Description
The sodium salt of Dithionite Na2S2O4 (Dit) is the 'zero oxygen solution powder' used for calibration of oxygen sensors at zero oxygen concentration, or for stepwise reduction of oxygen concentrations in instrumental O2 background tests. It is not recommended to use dithionite in experiments with biological samples or several multisensor approaches, for these see Setting the oxygen concentration.
Abbreviation: Dit
Reference: MiPNet06.03 POS-calibration-SOP, MiPNet14.06 Instrumental O2 background
Application in HRR
- Dit - (The abbreviation 'Dith' has been used previously and is stepwise replaced by Dit.): Dithionite (Sodium hydrosulfite; Na2S2O4), Sigma-Aldrich: 71699, store at 4 °C, CAS: 7775-14-6, M = 174.11 g·mol-1
- Hazard statements: H251, H302, H319; self-heating; may cause fire, harmful if swallowed, causes serious eye irritation
- Dit - (The abbreviation 'Dith' has been used previously and is stepwise replaced by Dit.): Dithionite (Sodium hydrosulfite; Na2S2O4), Sigma-Aldrich: 71699, store at 4 °C, CAS: 7775-14-6, M = 174.11 g·mol-1
- Note: Dithionite is oxidized when exposed to air. We recommend to reduce contact to air during preparation in a closed tube. The effective concentration of a solution of reduced dithionite is lower than the nominal concentration (30 mM or 10 mM) after dithionite has been partially oxidized in the powder or in aqueous solution.
- To calculate the injection volume of Na-dithionite solution to reduce O2 concentration to specific desired values, see Section 3.2 in MiPNet14.06 Instrumental O2 background.
- Note: Dithionite is oxidized when exposed to air. We recommend to reduce contact to air during preparation in a closed tube. The effective concentration of a solution of reduced dithionite is lower than the nominal concentration (30 mM or 10 mM) after dithionite has been partially oxidized in the powder or in aqueous solution.
- Preparation of 30 mM dithionite solution for instrumental oxygen background test (dissolved in phosphate buffer, 50 mM, pH 8)
- Weigh 0.051 g dithionite, sodium hydrosulfite, and transfer to 10 mL volumetric glass flask.
- Adjust final volume to 10 mL with Phosphate buffer and keep closed to avoid decomposing.
- Preparation of 10 mM dithionite solution for instrumental oxygen background test (dissolved in Phosphate buffer, 50 mM, pH 8)
- Weigh 0.017 g dithionite, sodium hydrosulfite, and transfer to 10 mL volumetric glass flask.
- Adjust final volume to 10 mL with Phosphate buffer and keep closed to avoid decomposing.
- Experiments performed with 10 and 30 mM dithionite solution showed identical results. We advise to use a concentration of 30 mM for performing the instrumental oxygen background test. For new dithionite, 10 mM dithionite solution may be used.
- Preparation of 2.5 mM dithionite solution for instrumental oxygen background test with the O2k-sV-Module (0.5 mL) (dissolved in phosphate buffer, 50 mM, pH 8)
- Weigh 0.00425 g sodium dithionite, sodium hydrosulfite, and transfer to 10 mL volumetric glass flask.
- Adjust final volume to 10 mL with phosphate buffer and keep closed to avoid decomposing.
- Experiments performed with 2.5 mM dithionite solution are recommended for the O2k-sV-Module only.
- From MiPNet06.03 POS-calibration-SOP (Version 18): Prepare "zero solution" by dissolving c. 20 mg sodium hydrosulfite (OroboPOS-Service Kit) or two tips of a spatula in 0.5 mL water. Mix in a small vial with minimum gas space. Use fresh. Dithionite may not work after prolonged storage.
Further information »Talk:Dithionite
MitoPedia O2k and high-resolution respirometry:
O2k-Open Support
Troubleshooting
Zero oxygen concentration reached but 'Instrumental O2 background_TIP2k.DLP' is not finished
- Customer ID: UK Loughborough Bailey S
Question:
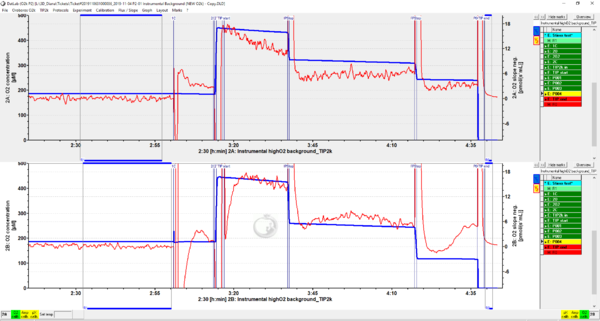
We have always had the same issue with our TIP2k running the background calibration that it has always reached ‘TIP2 end’ with the oxygen concentration reaching zero before the event P004 was activated meaning we are not able to get mark J°4h. The data is attached (2019-11-04).
Answer:
Stepwise analysis of the attached data shows the following:
Observations:
| Default parameters high O2 background TIP2K (.DLD) | ||
|---|---|---|
| Volume (nL) | Flow (nL/s) | |
| P1 | 100000 | 250 |
| P2 | 100000 | 250 |
| P3 | 50000 | 125 |
| P4 | 100000 | 50000 |
| Your high O2 background TIP2K (.DLD) | ||
| P1 | 100000 | 250 |
| P2 | 50000 | 125 |
| P3 | 100000 | 50000 |
| P4 | x | x |
- Please note that: On your protocol the event P2 has been missed (if you see, the default P3 and your P2 are the same steps);
A premise for P1 (please see on your DLD file under 'Feedback control' and after loading as protocol 'BG_Feedback-highO2_400-200') is to start when O2 concentration [µM] > 370 and to stop when O2 concentration [µM] < 360.
- However: Immediately after your P1 titration, you already have a O2 concentration [µM] = 322 and therefore your titration P1 goes on until the requirements for the next possible P titration are met;
The next possible titration requirements corresponds to P3 (in your DLD recognized as P2) (start when O2 concentration [µM] > 270 and to stop when O2 concentration [µM] < 260); Finally the P4 titration takes place (in your DLD recognized as P3) which corresponds to a 100 uL titration just before the event 'TIP end'.
- Conclusion: Since we observe that your O2 concentration decreases too fast at each Dit titration point, we can be sure that the Dit solution stock has an high concentration. Our recommendation for Dit concentration is between 10 and 30 mM. Due to the oxidation process that occurs when Dit is open for long time, we recommend to use a concentration of 30 mM for performing the instrumental oxygen background test, however in your case I advise you to use a lower concentration of your Dit solution.
During instrumental oxygen background test, the step representative of O2 consumption is too small after Dit titrations
- Customer ID: CH Lausanne Kayser B
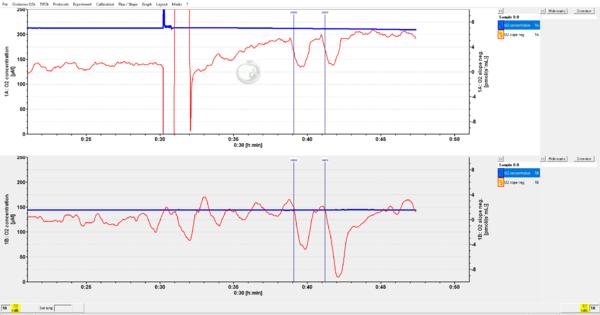
- Question: I ran a zero oxygen calibration after the instrumental background protocol and the O2 neg slope for O2 concentrations of 60 and 30 uM is positive and greater compared with the O2 neg slope at O2 concentrations of 180 and 120 uM. The data is attached (2019-09-11).
- Answer: Stepwise analysis of the attached data shows the following:
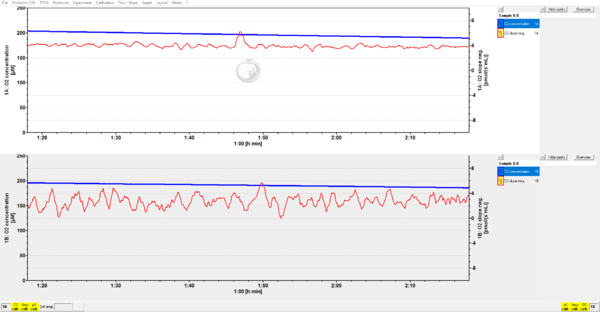
- Quality control (QC) 1: Your POS operates perfectly: during air calibration the O2 slope neg. approaches zero (see section 2.1 of https://wiki.oroboros.at/images/7/77/MiPNet06.03_POS-Calibration-SOP.pdf);
- Quality control (QC) 2: In the closed chamber, the O2 slope neg. is <4 pmol s-1 mL-1 which excludes any biological or chemical contamination from the MiR05 buffer. https://wiki.oroboros.at/images/6/65/MiPNet14.06_InstrumentalO2Background.pdf;
- From your dithionite titrations we can observed that:
- After titrations of 2.5 µL of Dit, the step representative of O2 consumption is too small – indicative of a low effective potency of the solution of Dit;
- At increasing concentrations of Dit towards stepwise lower O2 concentrations, we observe that the O2 slope negative does not decrease in a titration dependent manner. Therefore, O2 is being consumed by the solution even after the initial fast decline of oxygen. Since we excluded other potential problems, the irregular background traces are due to the Dit solution.(see representative trace on page 5 of https://wiki.oroboros.at/images/6/65/MiPNet14.06_InstrumentalO2Background.pdf)
- Taken together, your dithionite is highly oxidised and a continuing oxidation reaction explains the results.
- Solution: Assuming you prepared the Dit according to our SOP (always fresh, http://bioblast.at/index.php/Dithionite), I advise you to purchase a new Dit powder (your Dit may be old and/or has oxidized).
Anonymous user
Question:
Q1: The Oxygen Concentration couldn't go down while I tried zero oxygen calibration. No matter how much solution I added, the oxygen concentration still remain the same. I reassembled, but it still keeps the same. The data is attached.
Q2: The chamber B's oxygen slope is not stable. This is a new problem I have never seen. Please see the attachment.(2019-04-26)
Q3. The maximum Oxygen concentration of another Oroboros O2k is only about 140 μM. I don't how to increase the starting point of oxygen concentration.
Answer:
A1: I suspect that the dithionite solution was not correctly prepared or not fresh. Dithionite loses activity quite fast, so you need to make it freshly. Please check this site: http://bioblast.at/index.php/Dithionite
A2: While there is a difference between chambers A and B, the stability of the signal is still within the acceptable range of noise given by the specifications of the instrument, see: Oxygen_sensor_test.
A3: You may simply need to calibrate, please see here: http://wiki.oroboros.at/index.php/O2_calibration_-_DatLab
MitoPedia methods:
Respirometry
MitoPedia O2k and high-resolution respirometry:
O2k-Open Support
MitoPedia topics:
Substrate and metabolite