Doerrier 2018 MiP2018a
| Inter-laboratory harmonization of protocols for mitochondrial function evaluation in permeabilized muscle fibers. |
Link: MiP2018
Doerrier C, Gama-Perez P, Garcia-Roves P, Gnaiger E (2018)
Event: MiP2018
In recent years there has been an increasing interest in mitochondrial (mt) research in many physio-pathological conditions. In this context, the evaluation of mt-respiration is crucial for understanding mt-physiology and bioenergetics in health and disease. However, despite of the growing relevance in mt-research, there is no database which provides information about mt-function. MitoEAGLE network provides a powerful tool for establishing a useful mt-database in different species, tissues, and sample preparations. However, the comparison of reported data on mt-respiration from the scientific literature and inter/intra-laboratory comparisons are very difficult. In permeabilized muscle fibers (pfi) one of the critical issues comparing respiratory data is the quality of the mitochondrial preparations. Therefore, the generation of reference values which allow the evaluation of the skills preparing high-quality pfi, constitutes one of the main aims of the WG2 (muscle) in the MitoEAGLE project. For this purpose, as a first step, we performed a pilot study collecting mt-respiratory data (pfi from mouse soleus) in different laboratories following the same experimental procedure. In this previous pilot study, we observed heterogeneous results between the groups involved [1]. In the present study, we addressed some potential aspects which could trigger this variability.
Mitochondrial respiration was evaluated by high-resolution respirometry in pfi from soleus muscle from N=15 C57BL6/J male mice using the SUIT-008 O2 pfi D014 protocol (Fig. 1) [2]. Experiments were performed in parallel in the same laboratory by two researchers from different research groups.
Our results did not exhibit significant differences on mitochondrial respiration by comparing individual mechanical sample preparations and, the effect of chemicals prepared for this study in both laboratories. On the other hand, we compared the results obtained for each researcher in the former and present study. We detected significant differences in oxygen consumption between both studies. Our findings demonstrate two sources of variability in mitochondrial respiration. First, ADP storage (time and/or temperature) seems to be a critical aspect affecting oxygen fluxes. Wet weight is the other important issue that must be considered and well-explained for generating reliable results in the future.
The achievement of the reference values constitutes the starting point for obtaining reliable data in any project, avoiding an under/overestimation that could mask/influence relevant results in a study. This fact will contribute to decrease and maybe avoid non-reproducible data between different laboratories. Finally, this study will benefit the elaboration of a public mt-database in muscle permeabilized tissues.
• Bioblast editor: Plangger M, Kandolf G, Doerrier Velasco CA
• O2k-Network Lab: AT Innsbruck Oroboros, ES Barcelona Garcia-Roves PM
Affiliations and Support
- Doerrier C(1), Gama-Pérez P(2), García-Roves P(2,3), Gnaiger E(1,4)
- Oroboros Instruments, Innsbruck, Austria
- Dept Physiological Sciences, Univ Barcelona
- Bellvitge Biomedical Research Inst (IDIBELL); Spain
- Dept Visceral, Transplant Thoracic Surgery, Daniel Swarovski Research Lab, Medical Univ Innsbruck, Austria. - carolina.doerrier@oroboros.at
- Doerrier C(1), Gama-Pérez P(2), García-Roves P(2,3), Gnaiger E(1,4)
- Contribution to European Union Framework Programme Horizon 2020 COST Action CA15203 MitoEAGLE.
Figures
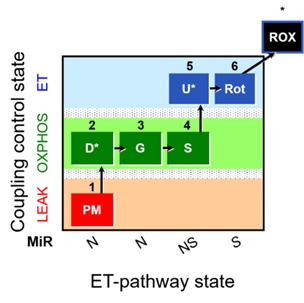
Figure 1. Representative diagram for the SUIT-008 O2 pfi D014 protocol. Briefly, 1PM, pyruvate and malate as substrates of NADH-linked pathway; 2D, saturated ADP to evaluate OXPHOS state in NADH-linked pathway; 2c, cytochrome c to assess the integrity of the outer mitochondrial membrane; 3G, glutamate for the NADH-pathway; 4S, succinate for NADH&Succinate pathway in OXPHOS state; 5U, uncoupler, for obtaining ET-state with NADH&Succinate linked-substrates; 6Rot, rotenone for inhibiting Complex I; 7Ama, antimycin A for blocking Complex III.
References
- Garcia-Roves Pablo M, Chabi B, Doerrier C, Dubouchaud H, Grefte S, Irving B, Ost M, Pesta D (2017) Mitochondrial respirometry reference values from permeabilized mouse soleus muscle fibers. MitoEAGLE WG2 pilot study. Garcia-Roves_2017_MiP2017
- SUIT-008_O2_pfi_D014
Labels: MiParea: Respiration
Organism: Mouse
Tissue;cell: Skeletal muscle
Preparation: Permeabilized tissue
Regulation: ADP, Flux control Coupling state: LEAK, OXPHOS, ET Pathway: N, S, NS, ROX HRR: Oxygraph-2k Event: Oral MitoEAGLE, Blebbistatin