Garcia-Roves 2019 MiPschool Coimbra
| Generating reference values on mitochondrial respiration in permeabilized muscle fibers. |
Link: MitoEAGLE
Garcia-Roves PM, Dahdah N, Gama-Perez P, Doerrier C, Gnaiger E, Lemieux H, Holody CD, Carpenter RG, Tepp K, Puurand M, Kaambre T, Dubouchaud H, Chabi B, Cortade F, Ost M, Pesta D, Calabria E, Casado M, Fernandez-Ortiz M, Acuna-Castroviejo D, Villena J, Grefte S, Keijer J, O'Brien KA, Sowton A, Murray AJ, Campbell MD, Marcinek DJ, Nollet E, Wuest R, Dayanidhi S (2019)
Event: MiPschool Coimbra 2019
As WG2 leader in the MitoEAGLE COST Action my main role is to stimulate, engage and coordinate scientists in the different tasks to achieve our proposed deliverables. In this specific WG we should deliver:
- A mitochondrial database on muscle tissue from humans and model organisms.
- A set of guidelines for future studies on mitochondrial bioenergetics in muscle tissue.
- Draft of review manuscripts on these topics.
In relation to MitoEAGLE data repository in muscle tissues, during my presentation I will summarize our progress in the generation of reference values for mitochondrial respirometry in permeabilized skeletal muscle sample preparations. The goal is that new researchers in the field follow a reference protocol and check if their values are in an acceptable range. This approach could serve to test researchers’ technical skills and therefore determine if they are proficient enough to perform their own experiments with confidence.
We initially ran a pilot study including 7 European groups. The results obtained show, as we expected, low variability among laboratories. After some adjustments and identifying some relevant steps that need to be carefully taken into consideration, the experimental procedure and results were judged satisfactory. Therefore, during Spring 2018 a new invitation was broadly open to laboratories worldwide. The final aim is to collect all the data and write a manuscript by 2020.
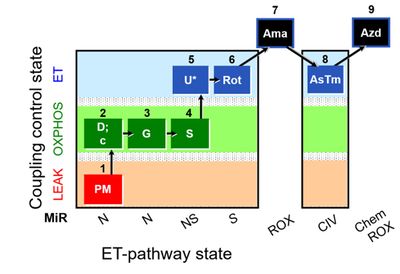
All research groups involved obtained respirometry reference values in permeabilized soleus fibers from males (N=4) and females (N=4) C57BL/6J mice aged 14-16 weeks. The substrate-uncoupler-inhibitor titration (SUIT) protocol used in the study is represented in Figure 1 [1]. The presentation will give detailed information on the experimental procedures followed to fulfill our objective. Finally, we will present and discuss our preliminary data, including results from the second phase where ten additional international laboratories have performed the experiments.
This unique international study has the ambition to significantly contribute to major aims and concerns in the scientific community, as stablished more precisely in the MitoEAGLE memorandum of understanding:
- Data sharing
- Intensify the dissemination of updated knowledge and know-how among the partners.
- Mapping mitochondrial physiology and pathology to develop a mitochondrial database.
- Reproducibility crisis
- MitoEAGLE recommendations: our goal is to increase the value and reduce the noise in mitochondrial research.
- Optimally harmonize protocols across research groups.
• Bioblast editor: Plangger M
Labels: MiParea: Respiration, Instruments;methods
Organism: Mouse
Tissue;cell: Skeletal muscle
Preparation: Permeabilized tissue
Coupling state: LEAK, OXPHOS, ET
Pathway: N, S, CIV, NS, ROX
HRR: Oxygraph-2k, O2k-Protocol
Affiliations and support
- Garcia-Roves PM (1), Gama-Perez P (1), Dahdah N (1), Doerrier C (2), Gnaiger E (2,3), Lemieux H (4), Holody CD (4), Carpenter RG (4), Tepp K (5), Puurand M (5), Kaambre T (5), Dubouchaud H (6), Chabi B (7), Cortade F (7), Ost M (8), Pesta D (9,10), Calabria E (11), Casado M (12), Fernandez-Ortiz M (13), Acuña-Castroviejo D (13), Villena JA (14), Grefte S (15), Keijer J (15), O'Brien K (16), Sowton A (16), Murray AJ (16), Campbell MD (17), Marcinek DJ (17), Nollet E (18), Wüst R (18), Dayanidhi S (19)
- Dept Physiological Sciences, Univ Barcelona Bellvitge Biomedical Research Inst (IDIBELL), Spain
- Oroboros Instruments, Innsbruck, Austria
- Dept Visceral, Transplant Thoracic Surgery, Daniel Swarovski Research Lab, Medical Univ Innsbruck, Austria
- Fac Saint-Jean, Univ Alberta, Canada
- Lab Chemical Biology, National Inst Chemical Physics Biophysics, Estonia
- DMEM, Univ Montpellier, INRA, France
- Lab Bioénergétique Fondamentale Appliquée, Univ Grenoble Alpes, INSERM, U1055, France
- German Inst Human Nutrition Potsdam-Rehbruecke, Germany
- Inst Clinical Diabetology, German Diabetes Center, Leibniz Center Diabetes Research Heinrich-Heine Univ Düsseldorf, Germany
- German Center Diabetes Research, Munich, Neuherberg, Germany
- Dept Neurological Movement Sciences, Univ Verona, Italy
- Dept Molecular Cellular Pathology Therapy, Inst Biomedicina Valencia, Spain
- Biomedical Research Center, Univ Granada, Spain
- Metabolism Obesity Lab, Vall d’Hebron Research Inst, Spain
- Human Animal Physiology, Wageningen Univ, The Netherlands
- Dept Physiology, Development & Neuroscience, Univ Cambridge, UK
- Dept Radiology, Univ Washington, South Lake Union, USA
- Dept Human Movement Sciences, Fac Behavioural Movement Sciences, Vrije Univ Amsterdam, The Netherlands
- Rehabilitation Inst Chicago, Feinberg School Medicine, Northwestern Univ, USA. - pgarciaroves@ub.edu
- Support: European Union Framework Programme Horizon 2020 COST Action CA15203 MitoEAGLE. Instituto de Salud Carlos III- reference PI15/00701. K-Regio project MitoFit. National Institute of Health-P01 AG001751.
Figures
Figure 1: SUIT protocol. Saturating concentrations of pyruvate&malate (1PM); ADP with MgCl2 (2D; kinetically saturating [ADP] must be tested in each experiment); cytochrome c (2c); glutamate (3G); succinate (4S; add 2.5 mM ADP to check if ADP is saturating after addition of succinate); FCCP/CCCP (5U*): 0.5 µM titration steps to reach maximum noncoupled respiration (ET), report the uncoupler used (FCCP or CCCP); rotenone (6Rot) for succinate-ET capacity; antimycin A (7Ama) for measurement of residual oxygen consumption (Rox). Optionally, CIV activity could be measured by using ascorbate and TMPD (8AsTm) and azide (9Azd). Oxygen concentration range in the experiment was maintained between 400-250 µM O2.
References
- Lemieux H, Blier PU, Gnaiger E (2017) Remodeling pathway control of mitochondrial respiratory capacity by temperature in mouse heart: electron flow through the Q-junction in permeabilized fibers. Sci Rep 7:2840, DOI:10.1038/s41598-017-02789-8. - SUIT-8_O2_pfi_D14