Mitchell 2011 Biochim Biophys Acta
| Mitchell P (1966) Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. https://doi.org/10.1016/j.bbabio.2011.09.018 |
» Biochim Biophys Acta Bioenergetics 1807 (2011):1507-38. Science Direct Open Access
Mitchell P (1966) Biochim Biophys Acta
Abstract: 50 years ago Peter Mitchell proposed the chemiosmotic hypothesis for which he was awarded the Nobel Prize for Chemistry in 1978. His comprehensive review on chemiosmotic coupling known as the first “Grey Book”, has been reprinted here with permission, to offer an electronic record and easy access to this important contribution to the biochemical literature. This remarkable account of Peter Mitchell's ideas originally published in 1966 is a landmark and must-read publication for any scientist in the field of bioenergetics. As far as was possible, the wording and format of the original publication have been retained. Some changes were required for consistency with BBA formats though these do not affect scientific meaning. A scanned version of the original publication is also provided as a downloadable file in Supplementary Information. See also Editorial in this issue by Peter R. Rich. Original title: CHEMIOSMOTIC COUPLING IN OXIDATIVE AND PHOTOSYNTHETIC PHOSPHORYLATION, by Peter Mitchell, Glynn Research Laboratories, Bodmin, Cornwall, England. • Keywords: Chemiosmotic theory, Proton pumping, Mitochondria, Respiration, Photosynthesis, Oxidative phosphorylation, Peter Mitchell • Bioblast editor: Gnaiger E
Force or pressure? - The linear flux-pressure law
- "For many decades the pressure-force confusion has blinded the most brilliant minds, reinforcing the expectation that Ohm’s linear flux-force law should apply to the hydrogen ion circuit and protonmotive force. .. Physicochemical principles explain the highly non-linear flux-force relation in the dependence of LEAK respiration on the pmF. The explanation is based on an extension of Fick’s law of diffusion and Einstein’s diffusion equation, representing protonmotive pressure ― isomorphic with mechanical pressure, hydrodynamic pressure, gas pressure, and osmotic pressure ― which collectively follow the generalized linear flux-pressure law."
- Gnaiger E (2020) Mitochondrial pathways and respiratory control. An introduction to OXPHOS analysis. 5th ed. Bioenerg Commun 2020.2. https://doi.org/10.26124/bec:2020-0002
- » pressure = force × free activity
Selected quotes
- The terms force and potential, versus pressure and concentration difference are emphasized in bold below, but not in the original.
- (p 15) ATP hydrolysis would be coupled to the translocation of OH- groups or ions across the system with a stoichiometry of one OH- translocated (equivalent to one proton translocated in the opposite direction) per ATP hydrolysed.
- (p 15/16) According to the more primitive form of the chemiosmotic coupling hypothesis, ATP hydro1sysis and substrate oxidation would each a difference of concentration of protons in the same direction across a proton-impermeable membrane, so that, if the proton concentration difference could become big enough, electron transport would reverse ATP hydrolysis, and ATP hydrolysis would exert a back pressure on electron transport.
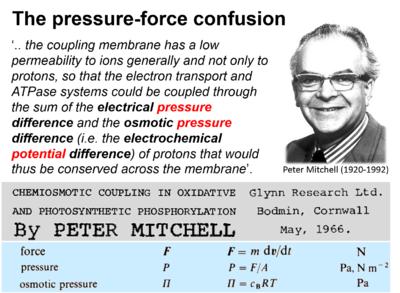
- (p 16) Unfortunately, the pH difference corresponding to the proton concentration difference required to reverse ATP hydrolysis through the ATPase system appears to be too large for this primitive protonmotive mechanism to work simply on the basis of the osmotic pressure of the protons. The mechanism was accordingly sophisticated by postulating that the coupling membrane has a low permeability to ions generally and not only to protons, so that the electron transport and ATPase systems could be coupled through the sum of the electrical pressure difference and the osmotic pressure difference (i.e. the electrochemical potential difference) of protons that would thus be conserved across the membrane. Under such conditions it would be possible for the major part of the electrochemical potential difference to be due to the membrane potential, and there need be only a relatively small pH difference (Mitchell, 1961a).
- (p 102) The proton-translocating respiratory chain and ATPase systems are assumed to be orientated in this membrane so that a displacement of protons through either system would enable the processes of oxido-reduction and phosphorylation to be coupled by the proton circuit operating at an effective pressure, or P.M.F., of some 250 mV.
- (p 108) In rat liver mitochondria, the volume of aqueous medium in the internal and external compartments each amount to very approximately 1 ml per g mitochondrial protein (Amoore & Bartley, 1958: Bartley, 1961: Bartley & Enser, 1964).
- (p 122/123) Owing to the restriction of the return flow of protons, the P.M.F. would be high and the back pressure on oxido-reduction would be sufficient to slow respiration to the "controlled" rate by the mechanism considered in Section IV(3).
- (p 126) We presume that the transition of the system from State 3, or its equivalent, to State 4, or its equivalent, occurs because the membrane potential builds up to the point at which it successfully balances the forward o/r or h/d chemical pressure.
- (164/165) (5) Osmotic pressure and water flow - As indicated in Section III(1), the ATPase II system would not actually transport protons inwards during ATP synthesis in mitochondria, but would transport 02- outwards. The effect of the outward transport of 02- would be equivalent to the translocation of 2H+ inwards and H20 outwards. Hence, in State 3, for example, water would be chemically transported outwards through the membrane. This water flux is not, however, likely to contribute significantly to the osmotic steady state of the mitochondria, because the rate of equilibration of water by diffusion across the coupling membrane would be expected to be high, compared with that of the main solutes present in the internal and external aqueous phases. Similar considerations would apply to water movement in chloroplasts and bacteria. Accordingly, the reversible o/r-linked and h/d-linked swelling and shrinkage changes of mitochondria, chloroplasts, and bacteria can probably be ascribed mainly to the flow of water, as such, under its own activity gradient, following
the metabolically linked translocation of osmotically active solutes.
Cited by
- Gnaiger E (2020) Mitochondrial pathways and respiratory control. An introduction to OXPHOS analysis. 5th ed. Bioenerg Commun 2020.2. https://doi.org/10.26124/bec:2020-0002
- Gnaiger E et al ― MitoEAGLE Task Group (2020) Mitochondrial physiology. Bioenerg Commun 2020.1. doi:10.26124/bec:2020-0001.v1.
Labels: MiParea: Respiration, mt-Awareness
Regulation: ATP production, Coupling efficiency;uncoupling, Q-junction effect
Ambiguity crisis, Made history, Protonmotive force, Force, Pressure, BEC 2020.1, BEC 2020.2