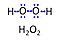NextGen-O2k
The revolutionary all-in-one instrument to conquer mitochondrial disease
- Oroboros - as a driving force in mitochondrial physiology - extends the analytical and diagnostic power of high-resolution respirometry by integration of NADH- and Q-redox monitoring in the NextGen-O2k. We aim at establishing the Oroboros quality control management for dissemination to our worldwide O2k-Network laboratories. This will become an effective contribution to address the acute reproducibility crisis of scientific investigation. In the spirit of Open Science and global networking, we will enable data sharing across projects and institutions in an Open Access database on mitochondrial physiology and pathology, to resolve the inflation crisis and ultimately the value-impact crisis of present academic publication. This will support key developments in mitochondrial medicine. In addition, we expand our business to algal biotechnology and ecology with the photobiology module of the NextGen-O2k, widening our focus from medicine to environment and climate.
- NextGen-O2k
- Extend high-resolution respirometry (HRR) to the all-in-one device
- The NextGen-O2k is a MultiSensor all-in-one instrument for in-depth studies of (1) the mitochondrial role in human diseases, and (2) mitochondria and chloroplasts ability in regulating algal growth and metabolite production rates.
- (+1) Detection of the redox state of the Q-junction and NAD-junction
- (+2) Controlled wavelengths and light intensity: PhotoBiology-Module
- Save on experimental time, reagents and samples
- Minimize errors and ensure reproducible and accurate results
- Highest oxygen sensitivity (5 nM detection limit)
- Exceptional resolution (wide oxygen concentration range, between 0 - 1,000 μM)
- Robustness (>10 years lifespan)
- The NextGen-O2k is a MultiSensor all-in-one instrument for in-depth studies of (1) the mitochondrial role in human diseases, and (2) mitochondria and chloroplasts ability in regulating algal growth and metabolite production rates.
- »Bioblast links: O2k-technology and HRR - >>>>>>> - Click on [Expand] or [Collapse] - >>>>>>>
- Oroboros Marketplace
- » O2k
- Oroboros Marketplace
- Concept
- » Concentration
- » Coupling-control state
- » Mitochondrial preparations
- » Oxidative phosphorylation
- » Oxygen, dioxygen, O2
- » Oxygen flux
- » Electron-transfer-pathway state
- » Respiration
- » Respirometry; » MitoPedia: Respirometry
- » Substrate-uncoupler-inhibitor titration (SUIT) protocols; » MitoPedia: SUIT
- Concept
NextGen-O2k publications, preprints, and abstracts
| Link | Reference | Year | View |
|---|---|---|---|
| Donnelly 2024 Redox Biol | Donnelly C, Komlódi T, Cecatto C, Cardoso LHD, Compagnion A-C, Matera A, Tavernari D, Campiche O, Paolicelli RC, Zanou N, Kayser B, Gnaiger E, Place N (2024) Functional hypoxia reduces mitochondrial calcium uptake. Redox Biol 71:103037. https://doi.org/10.1016/j.redox.2024.103037 | 2024 | PMID: 38401291 Open Access |
| Balmaceda 2024 Biochim Biophys Acta Mol Basis Dis | Balmaceda V, Komlodi T, Szibor M, Gnaiger E, Moore AL, Fernandez-Vizarra E, Viscomi C (2024) The striking differences in the bioenergetics of brain and liver mitochondria are enhanced in mitochondrial disease. Biochim Biophys Acta Mol Basis Dis 1870:167033. https://doi.org/10.1016/j.bbadis.2024.167033 | 2024 | PMID: 38280294 Bioblast link |
| Ravasz 2024 Sci Rep | Ravasz D, Bui D, Nazarian S, Pallag G, Karnok N, Roberts J, Marzullo BP, Tennant DA, Greenwood B, Kitayev A, Hill C, Komlódi T, Doerrier C, Cunatova K, Fernandez-Vizarra E, Gnaiger E, Kiebish Michael A, Raska A, Kolev K, Czumbel B, Narain NR, Seyfried TN, Chinopoulos C (2024) Residual Complex I activity and amphidirectional Complex II operation support glutamate catabolism through mtSLP in anoxia. Sci Rep 14:1729. https://doi.org/10.1038/s41598-024-51365-4 | 2024 | PMID: 38242919 Open Access |
| MiPNet28.07 NextGen-O2k Series XB manual | NextGen-O2k Series XB manual | 2023-10-19 | |
| Perin 2023 Proc Natl Acad Sci U S A | Perin G, Bellan A, Michelberger T, Lyska D, Wakao S, Niyogi KK, Morosinotto T (2023) Modulation of xanthophyll cycle impacts biomass productivity in the marine microalga Nannochloropsis. Proc Natl Acad Sci U S A 120:e2214119120. https://doi.org/10.1073/pnas.2214119120 | 2023 | PMID: 37307488 Open Access |
| Donnelly 2023 MitoFit | Donnelly C, Komlódi T, Cecatto C, Cardoso LHD, Compagnion AC, Matera A, Tavernari D, Zanou N, Kayser B, Gnaiger E, Place N (2023) Functional hypoxia reduces mitochondrial calcium uptake. MitoFit Preprints 2023.2. https://doi.org/10.26124/mitofit:2023-0002 — 2024-11-17 published in Redox Biol. | 2023 | MitoFit Preprints 2023.2. Functional hypoxia reduces mitochondrial calcium uptake. |
| MiPNet26.13 NextGen-O2k manual | NextGen-O2k manual | 2022-07-25 | |
| MiPNet26.12 NextGen-O2k: NADH-Module | NextGen-O2k: NADH-Module manual | 2022-05-17 | |
| Spielmann 2022 Mamm Genome | Spielmann N, Schenkl C, Komlódi T, da Silva-Buttkus P, Heyne E, Rohde J, Amarie OV, Rathkolb B, Gnaiger E, Doenst T, Fuchs H, Gailus-Durner V, de Angelis MH, Szibor M (2022) Knockout of the Complex III subunit Uqcrh causes bioenergetic impairment and cardiac contractile dysfunction. Mamm Genome 10.1007/s00335-022-09973-w | 2022 | Open Access PMID:36565314 |
| Pallag 2022 Int J Mol Sci | Pallag G, Nazarian S, Ravasz D, Bui D, Komlódi T, Doerrier C, Gnaiger E, Seyfried TN, Chinopoulos C (2022) Proline oxidation supports mitochondrial ATP production when Complex I is inhibited. https://doi.org/10.3390/ijms23095111 | 2022 | Int J Mol Sci 23:5111. PMID: 35563503 Open Access |
| Vera-Vives 2022 BEC | Vera-Vives AM, Perin G, Morosinotto T (2022) High-resolution photosynthesis-irradiance curves in microalgae. Bioenerg Commun 2022.19. https://doi.org/10.26124/bec:2022-0019 | 2022 | Bioenerg Commun 2022.19. published online 2022-12-15 |
| MiPNet24.12 NextGen-O2k: Q-Module | NextGen-O2k: Q-Module manual | 2021-10-29 | |
| MiPNet26.11 NextGen-O2k: PB-Module | NextGen-O2k: PB-Module manual | 2021-10-29 | |
| MiPNet24.16 DatLab8.0: CV-Module | DatLab8.0: Cyclic voltammetry manual | 2021-10-28 | |
| Went 2021 MitoFit PB | Went N, Di Marcello M, Gnaiger E (2021) Oxygen dependence of photosynthesis and light-enhanced dark respiration studied by High-Resolution PhotoRespirometry. MitoFit Preprints 2021.05. https://doi.org/10.26124/mitofit:2021-0005 | 2021 | MitoFit Preprints 2021.05. |
| Komlodi 2021 BEC Q | Komlódi T, Cardoso LHD, Doerrier C, Moore AL, Rich PR, Gnaiger E (2021) Coupling and pathway control of coenzyme Q redox state and respiration in isolated mitochondria. Bioenerg Commun 2021.3. https://doi.org/10.26124/bec:2021-0003 | 2021 | Bioenerg Commun 2021.3. |
| Cardoso 2021 BEC MgG | Cardoso LHD, Doerrier C, Gnaiger E (2021) Magnesium Green for fluorometric measurement of ATP production does not interfere with mitochondrial respiration. Bioenerg Commun 2021.1. https://doi.org/10.26124/bec:2021-0001 | 2021 | Bioenerg Commun 2021.1. |
| Copsey 2021 Sci Rep | Copsey AC, Barsottini MRO, May B, Xu F, Albury MS, Young L, Moore AL (2021) Kinetic characterisation and inhibitor sensitivity of Candida albicans and Candida auris recombinant AOX expressed in a self-assembled proteoliposome system. Sci Rep 11:14748. | 2021 | PMID: 34285303 Open Access |
| Went 2021 Batchelor thesis | Went N (2021) Oxygen dependence of photosynthesis and light-enhanced dark respiration studied by High-Resolution PhotoRespirometry. Batchelor thesis:26 pp. | 2021 | Went 2021 Batchelor thesis |
| Huete-Ortega 2020 MitoFit Preprint Arch EA | Huete-Ortega M, Di Marcello M, Iglesias-Gonzalez J, Gnaiger E (2020) High-resolution respirometry for chloroplast and mitochondrial bioenergetics in Chlamydomonas reinhardtii ― towards biotechnology exploitations. https://doi.org/10.26124/mitofit:ea20.algaeurope.0001 | 2020 | MitoFit Preprint Arch EA20.1. High-resolution respirometry for chloroplast and mitochondrial bioenergetics in Chlamydomonas reinhardtii ― towards biotechnology exploitations |
Support
- Supported by project NextGen-O2k which has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No. 859770.
MitoPedia: NextGen-O2k
- » NextGen-O2k«
| Term | Abbreviation | Description |
|---|---|---|
| Hydrogen peroxide | H2O2 | Hydrogen peroxide, H2O2 or dihydrogen dioxide, is one of several reactive oxygen intermediates generally referred to as reactive oxygen species (ROS). It is formed in various enzyme-catalyzed reactions (e.g., superoxide dismutase) with the potential to damage cellular molecules and structures. H2O2 is dismutated by catalase to water and oxygen. H2O2 is produced as a signaling molecule in aerobic metabolism and passes membranes more easily compared to other ROS. |
| Light-enhanced dark respiration | LEDR | Light-enhanced dark respiration LEDR is a sharp (negative) maximum of dark respiration in plants in response to illumination, measured immediately after switching off the light. LEDR is supported by respiratory substrates produced during photosynthesis and closely reflects light-enhanced photorespiration (Xue et al 1996). Based on this assumption, the total photosynthetic oxygen flux TP is calculated as the sum of the measured net photosynthetic oxygen flux NP plus the absolute value of LEDR. |
| Mitochondrial membrane potential | mtMP, ΔΨp+, ΔelFep+ [V] | The mitochondrial membrane potential difference, mtMP or ΔΨp+ = ΔelFep+, is the electric part of the protonmotive force, Δp = ΔmFeH+.
|
| NADH-Module | The NADH-Module, is a component of the NextGen-O2k for simultaneous measurement of oxygen consumption and NAD(P)H autofluorescence. NAD(P)H autofluorescence is used to evaluate the redox state of the NAD(P)H-pool. The NADH-Module incorporates an UV light and NADH-Sensors which include a photodiode and specific filters. | |
| NADH-Sensor | The NADH-Sensor has been developed as a part of the NADH-Module for simultaneous monitoring of oxygen consumption and NADH redox state. The NADH-Sensor is composed of a photodiode and equipped with three supergel R370 Italian blue filters (Rosco, US). | |
| NextGen-O2k | ||
| NextGen-O2k Technical developments | ||
| Oxygen kinetics | Oxygen kinetics describes the dependence of respiration of isolated mitochondria or cells on oxygen partial pressure. Frequently, a strictly hyperbolic kinetics is observed, with two parameters, the oxygen pressure at half-maximum flux, p50, and maximum flux, Jmax. The p50 is in the range of 0.2 to 0.8 kPa for cytochrome c oxidase, isolated mitochondria and small cells, strongly dependent on Jmax and coupling state. | |
| PB Light Source | The PhotoBiology Light Source (PBLS) has been designed as a part of the PB-Module to provide with an external source of light. This enables experiments for evaluating the production of O2 in the presence of light. The PBLS consists of one LED and one photodiode mounted on the PBLS head protected by a PMMA plastic cover. Three pairs of PBLS (white, blue, and red) are provided with the PB-Module. The light intensity can be regulated from 0 to 2750 µmol·s-1·m-2 (red PBLS), from 0 to 3000 µmol·s-1·m-2 (blue PBLS), from 0 to 3500 µmol·s-1·m-2 (white PBLS). An integrated photodiode provides real-time measurement of the light intensity allowing for continuous adjustment to the desired value. | |
| PB-Module | The PB-Module has been developed for conducting measurements of PhotoBiology, including photosynthesis. It consists of the PB Light Source and electronic components which are an integral part of the NextGen-O2k. Measurements are recorded and evaluated with the DatLab 8 software. | |
| PhotoBiology | PB | PhotoBiology is the science of the effect of light on biological processes. This includes photosynthesis, photochemistry, photophysics, photomorphogenesis, vision, bioluminescence, circadian rhythms and photodynamic therapy. Phototoxicity results from non-ionizing radiation (i.e. ultraviolet, visible and infrared radiation). Non-ionizing radiation is any type of electromagnetic radiation that does not carry enough energy per quantum (photon energy below 10 eV) to completely remove an electron from an atom or molecule. When photons interact with molecules, the molecules can absorb the photon energy and become excited, reacting with surrounding molecules and stimulating "photochemical" and "photophysical" changes. Respiration may be affected by light during photosynthesis or in dark respiration, with the transient response of light-enhanced dark respiration. |
| Photodecomposition | PD | Photodecomposition or photodegradation is the process of decay of organic material induced by increasing light intensity. Under aerobic conditions, the enhancement of photodecomposition by light intensity can be quantified by oxygen consumption in a controlled light regime. |
| Photosynthesis | PS | Photosynthesis is the process that converts light energy into chemical energy which is subsequently transformed to the physiological energy demand. Photosynthesis has a light-dependent and light-independent (dark) phase. In plants, algae, and cynobacteria, light energy is absorbed during the light phase by the pigment chlorophyll and used to split water and generate adenosine triphosphate (ATP) and reducing power - nicotinamide adenine dinucleotide phosphate (NADPH), with the net production of O2 as a waste product. During the dark phase ATP and NADPH are used to synthesize carbohydrates from CO2 through the metabolic pathway called Calvin-Benson cycle. Oxygenic photosynthesis is responsible for producing and maintaining the oxygen concentration of the Earth’s atmosphere. In bacteria such as cyanobacteria, photosynthesis involves the plasma membrane and the cytoplasm. In eukaryotic cells (plants and algae), photosynthesis takes place in the chloroplasts. |
| Q-Module | Q-Module | The Q-Module, developed for measuring the Q redox state and cyclic voltammetry, is supported by the NextGen-O2k and consists of the Q-Sensor, integrated electronic components in the O2k, and the DatLab software. |
| Q-Sensor | The Q-Sensor has been designed as a part of the Q-Module for measurements with cyclic voltammetry and voltammetry, allowing for analysis of the Q redox state. The Q-Stopper with the reference electrode is called Q-Sensor, which is plugged in the NextGen-O2k. A three-electrode system is used to detect the Q redox state. Two of the three electrodes (glassy carbon and platinum electrode) are built into the Q-Stopper, while the reference electrode is removable (Reference-Electrode\2.4 mm). |
- Bioblast links: Q - >>>>>>> - Click on [Expand] or [Collapse] - >>>>>>>
- Coenzyme Q
- » Coenzyme Q
- » Quinone, Ubiquinone Q; oxidized
- » Quinol, Ubiquinol QH2; reduced
- » Semiquinone
- » Coenzyme Q2
- » Q-redox state
- » Q-pools
- Coenzyme Q
- Mitochondrial pathways, respiratory Complexes, and Q
- » Q-cycle
- » Q-junction
- » Convergent electron flow
- » NS-pathway
- » FNS
- » FNSGp
- Mitochondrial pathways, respiratory Complexes, and Q
- NextGen-O2k and Q-Module
- Bioblast links: NADH - >>>>>>> - Click on [Expand] or [Collapse] - >>>>>>>
Mitochondrial NADH and respiration
Mitochondrial NADH production
NextGen-O2k
- Bioblast links: Hydrogen peroxide - >>>>>>> - Click on [Expand] or [Collapse] - >>>>>>>
- Specific
- General
- Bioblast links: ATP production - >>>>>>> - Click on [Expand] or [Collapse] - >>>>>>>
Phosphorylation pathway
- Phosphorylation pathway substrates
- » ADP
- » ATP
- » Inorganic phosphate
- Phosphorylation pathway substrates
- Phosphorylation pathway inhibitors
Coupling control
Respiratory complexes and coupling
ATP production measurement
- Bioblast links: pH and protons - >>>>>>> - Click on [Expand] or [Collapse] - >>>>>>>
- pH and protons
- » pH
- » hydrogen ion H+
- » hydron H+
- » hydronium ion H3O+
- » hydride H-
- » proton p+
- » pH buffering capacity
- » proton flux
- » proton pump versus hydrogen ion pump
- » proton leak
- » proton slip
- » protonmotive force
- pH and protons
- O2k-pH
- » O2k-Catalogue: O2k-pH ISE-Module
- » O2k-Manual pH electrode: MiPNet23.15 O2k-pH ISE-Module
- » O2k-SOP: MiPNet08.16 pH calibration
- » File:PH-Calibration-List.xls
- » NextGen-O2k, ratiometric: Carboxy SNARF 1
- » NextGen-O2k, ratiometric: HPTS
- » pH calibration buffers
- O2k-pH
- O2k-Publications
- HRFR - general
- » O2k-Manual: MiPNet22.11 O2k-FluoRespirometer manual
- » O2k signals and output
- » O2k-SOP: MiPNet14.06 Instrumental O2 background
- » MiPNet19.18A O2k-Series G: Start
- » ESD
- » O2k configuration
- » O2k control
- » O2k-FluoRespirometer
- » O2k-Main Unit#O2k-Series
- » Titration-Injection microPump
- » Compare: O2k-TPP+_ISE-Module
- HRFR - general
- DatLab
- Bioblast links: Calcium - >>>>>>> - Click on [Expand] or [Collapse] - >>>>>>>
- Inhibitors
- General
- Bioblast links: PhotoBiology and plant physiology - >>>>>>> - Click on [Expand] or [Collapse] - >>>>>>>
PhotoBiology: photosynthesis
Plant physiology: respiration
NextGen-O2k and PB-Module
- » NextGen-O2k
- » PB-Module
- » PB-Sensor
Labels:
MitoPedia:NextGen-O2k