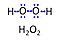NextGen-O2k Technical developments
The revolutionary all-in-one instrument to conquer mitochondrial disease.
WP1: Technical developments
Abstract
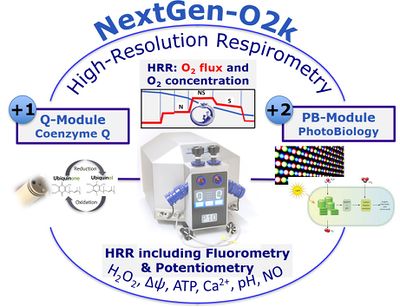
- Development of the Q-Module, NADH-Module, and PB-Module and integration in the NextGen-O2k
- Testing of the NextGen-O2k in collaboration with the Early testers
- Testing and development of the software for the NextGen-O2k Modules
- Integration of the new protocols in the DatLab software
NextGen-O2k: Horizon 2020 Framework Programme
- EIC-SMEInst-2018-2020 — SME instrument phase 2, Grant Agreement No. 859770, Oroboros Instruments
- Budget: € 2.35 Million
- Duration: 24 months, extended additional 5 months
- Application: 2019-01-09; Pitch in Brussels: 2019-02-15; Granted: 2019-03-05; Start: 2019-06-01 End: 2021-10-31
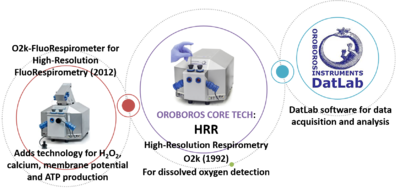
- With the O2k core technology, developed exclusively by Oroboros for high-resolution measurement of oxygen flux, the NextGen-O2k was developed.
- O2k
- High-resolution respirometry (HRR) and unique combinations of the O2k with
- Fluorometry: the O2k-FluoRespirometer
- Potentiometry: the O2k-pH ISE-Module and O2k-TPP+ ISE-Module
- Amperometric measurement of nitric oxide: the O2k-NO Amp-Module
Aims
- Form the O2k to the NextGen-O2k
- Between 1992 and 1994, Oroboros Instruments released its core technology for high-resolution respirometry (HRR), the Oxygraph-2k or O2k, for real-time monitoring of oxygen consumption of mitochondria, tissues, and living cells. Dissolved oxygen is measured using high-resolution polarographic oxygen sensors. Its main advantages are the unique signal stability and resolution of oxygen. HRR is the core technology present in every single of the devices and it will remain the basis of the NextGen-O2k.
- In 2012, the updated version of the O2k was launched, the O2k-FluoRespirometer. The integrated Smart Fluo-Sensors combine fluorescence excitation and detection of defined wavelengths that in combination with fluorophores allow for the measurement of additional mitochondrial parameters, such as membrane potential, H2O2 production, calcium concentration, and ATP production.
- The unique software DatLab has been developed for data acquisition and analysis. DatLab allows the researcher to perform calibrations (including automatic barometric and temperature adjustments), background correction, experimental SUIT (substrate-uncoupler-inhibitor-titration) protocols and data analysis.
Objectives
- The overall goal of this Phase 2 project is to complete the technical and scientific development of the new NextGen-O2k and prepare it for market launch.
- WP1
- » Technical developments
- WP2
- » Scientific developments
- WP3
- » Documentation
- WP4
- » Exploitation
- WP5
- » Dissemination
- WP6
- » Project management
Partners
- Our partners WGT-Elektronik and HTech have been essential in the development of the NextGen-O2k.
Early testers
- The influence of KOLs and Early testers/adopters will be used to build awareness of NextGen-O2k among researchers, academic and industrial partners. We have confirmed relevant mitochondrial and algal biotechnology researchers who want to collaborate as early testers:
Biomedical research Algal research Prof. Massimo Zeviani a,b, Dr. Carlo Viscomi c, Prof. Mervyn Singer a
a Medical Research Council - Mitochondrial Biology Unit, University of Cambridge, UK
b Department of Neurosciences, University of Padova, IT
c Department of Biomedical Sciences, University of Padova, ITDr. Tomas Morosinotto
Department of Biology, University of Padova, IT
Prof. Pablo Garcia-Roves
Department of Physiological Sciences, Faculty of Medicine and Health Sciences, University of Barcelona and Bellvitge Biomedical Research Institute (IDIBELL), ES
Prof. Yagut Allahverdiyeva-Rinne
Faculty of Technology, University of Turku, FI
Dr. Anthony Molina
Division of Geriatrics and Gerontology, Department of Medicine, University of San Diego, US
Prof. Claire Remacle
Genetics and Physiology of Microalgae, InBios/Phytosystems Research Unit, University of Liege, BE
Dr. Marten Szibor
Clinic for Heart and Thorax Surgery, Jena University Hospital, DE
Prof. Anthony Moore
Biochemistry and Medicine, School of Life Sciences, University of Sussex, UK
Dr. Christos Chinopoulos
Department of Medical Biochemistry, Semmelweis University, HU
NextGen-O2k events
| When | Where | |
|---|---|---|
| MiPNet28.12 IOC167 Schroecken AT | 2024-09-30 | Schroecken AT, 2024 Sep 30 - Oct 05. Oroboros O2k-Workshop on high-resolution respirometry (HRR), IOC167. |
| IOC166 Ljubljana SI | 2024-09-22 | Ljubljana SI, 2024 Sep 22 - Sep 26. Satellite symposium and workshop "Skeletal Muscle Research – from Cell to Human", IOC166. |
| MiPNet28.13 IOC164 Innsbruck AT | 2024-09-02 | Innsbruck AT, 2024 Sep 02-04. EBEC2024 Satellite Oroboros O2k-Workshop: Mito&Chlora High-Resolution Respirometry and PhotoBiology, IOC164. |
| MiPNet28.11 IOC163 Schroecken AT | 2024-06-17 | Schroecken AT, 2024 Jun 17-22. Oroboros O2k-Workshop on high-resolution respirometry (HRR), IOC163. |
| MiPNet28.02 IOC162 Schroecken AT | 2023-10-02 | Schroecken AT, 2023 Oct 02-07. Oroboros O2k-Workshop on high-resolution respirometry (HRR), IOC162. |
| MiPNet28.01 IOC160 Schroecken AT | 2023-06-19 | Schroecken AT, 2023 Jun 19-24. Oroboros O2k-Workshop on high-resolution respirometry (HRR), IOC160. |
| MiPNet28.05 IOC158 Innsbruck AT | 2023-02-27 | Innsbruck AT, 2023 Feb 27 - Mar 01. O2k-Coaching Days - Basic, IOC158. |
| Virtual OroDM02 | 2023 | Virtual Oroboros Distributor Meeting, OroDM02 |
| MiPNet27.04 IOC155 Schroecken AT | 2022-10-03 | Schroecken AT, 2022 Oct 03-08. Oroboros O2k-Workshop on high-resolution respirometry (HRR), IOC155. |
| MiPNet27.05 Schroecken BEC tutorial-Living Communications pmP | 2022-09-30 | Schroecken AT, 2022 Sep 30-Oct 03. BEC tutorial-Living Communications: pmF to pmP — pre IOC155. |
| MiPNet27.03 IOC154 Innsbruck AT | 2022-08-01 | Innsbruck AT, 2022 Aug 01-05. O2k-Coaching Days - O2k-FluoRespirometer and NextGen-O2k, IOC154. |
| MiPNet27.02 IOC153 Innsbruck AT | 2022-07-01 | Innsbruck AT, 2022 Jul 01-02. NextGen-O2k All-in-one Demo Workshop IOC153. |
| MiPNet 26.16 NextGen-O2k Summit 2021 Virtual | 2021-10-20 | Virtual, 2021 Oct 20. NextGen-O2k Summit, NextGen-O2k SME2. MiPNet 26.16. |
| The 4th Academic Symposium of Metabolic Biology Branch 2021 Guangxi CN | 2021-07-29 | Guangxi Province , CN, 2021 Jul 29 - Aug 02. The 4th Academic Symposium of Metabolic Biology Branch of Chinese Biophysical Society. |
| 19th Beijing Conference and Exhibition on Instrumental Analysis 2021 Beijing CN | 2021-07-27 | Beijing, CN, 2021 Sep 27-29. 19th Beijing Conference and Exhibition on Instrumental Analysis. |
| 19th Chinese Biophysics congress 2021 Anhui CN | 2021-07-23 | Anhui Province , CN, 2021 Jul 23-26. 19th Chinese Biophysics congress. |
| MiPNet 26.15 NextGen-O2k Event Photobiology: Algal bioenergetics Innsbruck AT | 2021-07-15 | Innsbruck AT, 2021 Jul 15-16. NextGen-O2k Event Photobiology: Algal bioenergetics. MiPNet 26.15. |
| 4th China Symposium on Nerve Control Metabolism 2021 Hangzhou city CN | 2021-06-18 | Hangzhou city, CN, 2021 Jun 18-19. The 4th China Symposium on Neuro-Controlled Metabolism. |
| World summit on Diabetes 2021 Virtual | 2021-06-16 | Vitual, 2021 Jun 16-17. World Summit on Diabetes: New technologies and practical approaches: Diabetes and Endocrine disorders |
| ESCI 2021 Virtual | 2021-06-09 | Virtual, 2021 Jun 9-11. 55th Annual Scientific Meeting of the European Society for Clinical Investigation, ESCI 2021. |
| 4th edition Metabolism & Cancer 2021 Virtual | 2021-05-27 | Virtual, 2021 May 27-29. 4th edition Metabolism & Cancer. |
| 24th Kalorimetrietage 2021 Braunschweig DE | 2021-05-26 | Braunschweig, DE, 2021 May 26-28. 24th Kalorimetrietage |
| ISAP 2021 Virtual | 2021-05-14 | Virtual, 2021 May 14-Aug 13. 7th Conference of the International Society for Applied Phycology - ISAP2021. |
| Career Event - FH Campus 2021 Wien AT | 2021-01-26 | Virtual, 2021 Jan 26. Career Event of the FH Campus Wien. |
| AlgaEurope 2020 Virtual Event | 2020-12-01 | Rome, IT, 2020 Dec 1-5. AlgaEurope 2020. |
| 2020 PaduaMuscleDays Padua IT | 2020-11-19 | Virtual, 2020 Nov 19-21. PaduaMuscleDays - 30 years of translational research |
| Analytica China 2020 Shanghai CN | 2020-11-16 | Shanghai, CN, 2020 Nov 16-18.Analytica China Exhibition - 10th International Trade Fair for Laboratory Technology, Analysis, Biotechnology and Diagnostics. |
| Long Night of Research 2020 Virtual Event | 2020-10-09 | Virtual Event, 2020 Oct 09. Oroboros at Long Night of Research, CCB. The diagnostic bioenergetic report – a milestone on the way to mitochondrial fitness and physical well-being. |
| 12th ÖGMBT Annual Meeting 2020 Virtual Event | 2020-09-21 | Virtual Event, 2020 Sep 21-23. 12th ÖGMBT Annual Meeting - Biomolecules in/for 21st century |
| MitoEAGLE WG2 Innsbruck AT | 2020-03-09 | Innsbruck AT, 2020 Mar 9-13. WG 2 on human skeletal muscle fibers retreat - COST Action MitoEAGLE. |
| MitoEAGLE WG4 Innsbruck AT | 2020-02-26 | Innsbruck AT, 2020 Feb 26-28. WG 4 blood cell workshop and retreat - COST Action MitoEAGLE. |
| MiPNet25.10 Distributor engagement Tokyo JP | 2020-02-04 | Tokyo,JP, 2020 Feb 4-7. On-site O2k-Workshop on High-Resolution Respirometry and partner distribution engagement. |
| MiPNet25.06 IOC145 Innsbruck AT | 2020-01-27 | Innsbruck AT, 2020 Jan 27-29. O2k-Coaching Days - Basic IOC145. |
| MiPNet25.03 IOC144 Innsbruck AT | 2020-01-20 | Innsbruck AT, 2020 Jan 20-22. O2k-Coaching Days - Basic IOC144. |
| 1st Myocardial Function Symposium 2020 Graz AT | 2020-01-10 | Graz, AT, 2020 Jan 10-11. 1st Myocardial Function Symposium: “Targets in cardiometabolic disease”. |
| NextGen-O2k SME2 coaching Orily Pratt part 2 | 2019-12-03 | Innsbruck AT, 2019 Dec 03-04 NextGen-O2k SME2 coaching Orily Pratt (part 2), NextGen-O2k SME2. |
| MiPNet24.15 IOC143 Haryana IN | 2019-11-15 | Haryana IN, 2019 Nov 15. Oroboros O2k-Workshop on high-resolution respirometry: Assessment of mitochondrial function in health and disease IOC143. |
| Neurocon 2019 Haryana IN | 2019-11-15 | Haryana, IN. 2019 Nov 15-18, Neurocon 2019. |
| MiP2019/MitoEAGLE Belgrade RS | 2019-10-13 | Belgrade RS, 13-16 Oct 2019. 14th Conference on Mitochondrial Physiology: Mitochondrial function: changes during life cycle and in noncommunicable diseases - COST MitoEAGLE perspectives and MitoEAGLE WG and MC Meeting. |
| MiPNet24.13 IOC142 Fukuoka JP | 2019-10-03 | ASMRM & J-mit 2019, Fukuoka JP, 2019 Oct 03 Oroboros Lunch Seminar at ASMRM & J-mit 2019 Fukuoka JP - Explore the Mito-World! (IOC142). |
| ASMRM & J-mit 2019 Fukuoka JP | 2019-10-03 | Fukuoka, JP, 2019 Oct 03-05. Joint ASMRM and J-mit Conference |
| MiPNet24.02 IOC141 Schroecken AT | 2019-09-23 | Schroecken AT, 2019 Sep 23-28 Oroboros O2k-Workshop on high-resolution respirometry (HRR), IOC141. |
| NextGen-O2k SME2 coaching Samia Kappe part 1 | 2019-09-12 | Innsbruck AT, 2019 Sep 10-11 NextGen-O2k SME2 coaching Samia Kappe (part 1), NextGen-O2k SME2. |
| FEBS Workshop Ageing 2019 Innsbruck AT | 2019-09-09 | Innsbruck, AT. 2019 Sep 09-12, FEBS Workshop “Ageing and Regeneration”. |
| Mitochondrial Physiology ‐ from Organelle to Organism 2019 Copenhagen DK | 2019-08-19 | Copenhagen DK , 2019 Aug 19-23. Mitochondrial Physiology ‐ from Organelle to Organism |
| NextGen-O2k SME2 coaching Orily Pratt part 1 | 2019-07-22 | Innsbruck AT, 2019 Jul 22-23 NextGen-O2k SME2 coaching Orily Pratt (part 1), NextGen-O2k SME2. |
| MiPschool Coimbra 2019 | 2019-07-08 | Coimbra, PT. 2019 Jul 08-11, 12th MiP/MitoEAGLE Training School 2019. Mitochondrial respiratory physiology: Challenges on data sharing, reproducibility, and interpretation. |
| OroDM01 Innsbruck AT | 2019-07-01 | Innsbruck AT, 2019 Jul 01-03 Oroboros Distributor Meeting, OroDM01. |
| MiPNet24.04 IOC140 Amsterdam NL | 2019-06-23 | Amsterdam NL, 2019 Jun 23 Pre-conference Oroboros O2k-Workshop on high-resolution respirometry (HRR), IOC140. |
| MiPNet24.01 IOC139 Schroecken AT | 2019-06-17 | Schroecken AT, 2019 June 17-22 Oroboros O2k-Workshop on high-resolution respirometry (HRR), IOC139. |
NextGen-O2k dissemination
- Cluster Award 2019
- The NextGen-O2k was awarded as the best Tyrolean innovation of the year 2019 with the Cluster Award in the category Life Science. The Cluster Award is funded by the Standortagentur Tirol.
- NextGen-O2k press releases
- NextGen-O2k ermöglicht noch präzisere Echtzeitmessung der Photosynthese in Algen - WKO online, 2022-08-28
- World Health Day: Discover how early diagnoses, virtual platforms and AI are improving our wellness - EIC community stories - online, 2022-04-07
- Technical leap in mitochondrial research and applications - CORDIS EU research results - online, 2022-03-21
- Tiroler Innovationspreis - Nominierter Technische Innovation - WKO Tirol, 2022-03-02
- Tiroler Innovationspreis geht in die nächste Runde, Tiroler Wirtschaft online, 2022-02-11
- Depression als Art 'Burnout' in den Zellen - Tiroler Tageszeitung online, Sep 2020, 2020-09-24
- Rekord bei Patenten, Tirol auf Rang sechs - Tiroler Tageszeitung, July 2020, 2020-07-03
- Oroboros Instrument revolutioniert Zellbiologische Wissenschaft - www.wirtschaftszeit.at; 2020-03-30
- Beste Innovationen aus Tirol] www.economyaustria.at, 2019-12-16
- Clusterawards 2019: Ausgezeichnete Innovationen aus Tirol science.apa.at, 2019-12-13
- Tiroler Unternehmen für Innovationen mit Clusterawards 2019 ausgezeichnet - www.wirtschaftszeit.at; 2019-12-16
- Clusterawards 2019: Ausgezeichnete Innovationen aus Tirol www.meinbezirk.at, 2019-12-13
- Tiroler Forschungsunternehmen startet durch - Tiroler Landeszeitung, September 2019, S30-31, 2019-09-23
- Innovationsförderung Tirol - Oroboros erhält Zuschuss in Millionenhöhe, Der Report 2019, Sonderheft der Wirtschaftsnachrichten, S12-13, 2019-09-18
- Dem Leben beim Atmen zusehen Der Standard, Beilage Forschung spezial, S 13, 2019-08-07.
- Turbo für Mitochondrienforschung und Oroboros Instruments, Standortagentur Tirol online, 2019-07-29
- Tiroler Unternehmen Oroboros erhält 1.7 Millionen Euro EU-Förderung, tt.com, 2019-07-29
- Turbo für Mitochondrienforschung udn Oroboros Instruments science.apa.at, online, 2019-07-29
- 1.7 Millionen Euro Förderung für Oroboros Instruments www.meinbezirk.at online, 2019-07-29
- 1,7 Millionen Euro EU-Flörderung für Tiroler KMU www.derbrutkasten.com online, 2019-07-29
- Tiroler Erfindung macht in Europa Furore theworldnews.net online, 2019-07-29
- Tiroler Erfindung macht in Europa Furore www.krone.at online, 2019-07-29
- EUR 1,7 Mio Förderung für Tiroler KMU Oroboros Tiroler Tageszeitung, S 16, 2019-07-30
- Tiroler Millionen-Erfindung Tiroler Krone, S 22, 2019-07-30
- NextGen-O2k press releases
- NextGen-O2k Literature Package - >>>>>>> - Click on [Expand] or [Collapse] - >>>>>>>
Q1: Are you satisfied with the material you received
- The following result emerged from all of the feedback responses received:
- Very satisfied: 71%
- Satisfied: 29%
- Neutral: 0%
- Dissatisfied: 0%
- Very dissatisfied: 0%
- The following result emerged from all of the feedback responses received:
Q2: Feel free to give your feedback
- Thanks to the interesting material. I will read it very carefully.
- Very useful! Thank you for sharing your research.
MitoEAGLE Q-workshop
Achievements
- Project Year 1: In the first year of the NextGen-O2k project, the new Q- and PB-Modules supported by the new architecture of the upgraded DatLab software have been developed and integrated. Additionally, we have successfully partnered with Key Opinion Leaders to begin testing the Q-Module prototypes. Working closely with our subcontractors WGT and H-Tech, key components such as cyclic voltammetry have been integrated into the system.
- Project Year 2: During the second year of the project the testing of the PB-Module by algae KOLs began, while testing of the Q-Module continued. Feedback prompted a change in the PB-Sensor that will allow a wider spectrum of research questions to be addressed. Features of the DatLab 8 software were implemented and tested, and SUIT protocols were integrated.
Progress and next steps
See » Track record
Some of the Key Opinion Leader’s at the IOC141 Sept. 2019
Gentle Science
- In addition to O2k-Workshops and on-site training, Oroboros will disseminate the not-for-profit initiatives on Mitochondrial Physiology and Bioenergetics Science Camps, specialized Mitochondrial Physiology Conferences, and Preprint publications:
- » Oroboros is a key player in support of the COST Action MitoEAGLE
- » MitoGlobal - the world-wide platform for societies and organizations supporting mitochondrial research and medicine
- » MitoEAGLE with cooperation by Oroboros launched MitoFit Preprints - the preprint server for mitochondrial physiology and bioenergetics
- » A vision on preprints
- » Mitochondrial respiratory states and rates : >530 coauthors join forces towards harmonization on nomenclature
- » Gentle Science - recognizes the responsibility of the scientific community - for the quality of science, the quality of life in science, and its mission
- In addition to O2k-Workshops and on-site training, Oroboros will disseminate the not-for-profit initiatives on Mitochondrial Physiology and Bioenergetics Science Camps, specialized Mitochondrial Physiology Conferences, and Preprint publications:
- »Bioblast links: O2k-technology and HRR - >>>>>>> - Click on [Expand] or [Collapse] - >>>>>>>
- Oroboros Marketplace
- » O2k
- Oroboros Marketplace
- Concept
- » Concentration
- » Coupling-control state
- » Mitochondrial preparations
- » Oxidative phosphorylation
- » Oxygen, dioxygen, O2
- » Oxygen flux
- » Electron-transfer-pathway state
- » Respiration
- » Respirometry; » MitoPedia: Respirometry
- » Substrate-uncoupler-inhibitor titration (SUIT) protocols; » MitoPedia: SUIT
- Concept
MitoPedia: NextGen-O2k
- » NextGen-O2k«
| Term | Abbreviation | Description |
|---|---|---|
| Hydrogen peroxide | H2O2 | Hydrogen peroxide, H2O2 or dihydrogen dioxide, is one of several reactive oxygen intermediates generally referred to as reactive oxygen species (ROS). It is formed in various enzyme-catalyzed reactions (e.g., superoxide dismutase) with the potential to damage cellular molecules and structures. H2O2 is dismutated by catalase to water and oxygen. H2O2 is produced as a signaling molecule in aerobic metabolism and passes membranes more easily compared to other ROS. |
| Light-enhanced dark respiration | LEDR | Light-enhanced dark respiration LEDR is a sharp (negative) maximum of dark respiration in plants in response to illumination, measured immediately after switching off the light. LEDR is supported by respiratory substrates produced during photosynthesis and closely reflects light-enhanced photorespiration (Xue et al 1996). Based on this assumption, the total photosynthetic oxygen flux TP is calculated as the sum of the measured net photosynthetic oxygen flux NP plus the absolute value of LEDR. |
| Mitochondrial membrane potential | mtMP, ΔΨp+, ΔelFep+ [V] | The mitochondrial membrane potential difference, mtMP or ΔΨp+ = ΔelFep+, is the electric part of the protonmotive force, Δp = ΔmFeH+.
|
| NADH-Module | The NADH-Module, is a component of the NextGen-O2k for simultaneous measurement of oxygen consumption and NAD(P)H autofluorescence. NAD(P)H autofluorescence is used to evaluate the redox state of the NAD(P)H-pool. The NADH-Module incorporates an UV light and NADH-Sensors which include a photodiode and specific filters. | |
| NADH-Sensor | The NADH-Sensor has been developed as a part of the NADH-Module for simultaneous monitoring of oxygen consumption and NADH redox state. The NADH-Sensor is composed of a photodiode and equipped with three supergel R370 Italian blue filters (Rosco, US). | |
| NextGen-O2k | ||
| NextGen-O2k Technical developments | ||
| Oxygen kinetics | Oxygen kinetics describes the dependence of respiration of isolated mitochondria or cells on oxygen partial pressure. Frequently, a strictly hyperbolic kinetics is observed, with two parameters, the oxygen pressure at half-maximum flux, p50, and maximum flux, Jmax. The p50 is in the range of 0.2 to 0.8 kPa for cytochrome c oxidase, isolated mitochondria and small cells, strongly dependent on Jmax and coupling state. | |
| PB Light Source | The PhotoBiology Light Source (PBLS) has been designed as a part of the PB-Module to provide with an external source of light. This enables experiments for evaluating the production of O2 in the presence of light. The PBLS consists of one LED and one photodiode mounted on the PBLS head protected by a PMMA plastic cover. Three pairs of PBLS (white, blue, and red) are provided with the PB-Module. The light intensity can be regulated from 0 to 2750 µmol·s-1·m-2 (red PBLS), from 0 to 3000 µmol·s-1·m-2 (blue PBLS), from 0 to 3500 µmol·s-1·m-2 (white PBLS). An integrated photodiode provides real-time measurement of the light intensity allowing for continuous adjustment to the desired value. | |
| PB-Module | The PB-Module has been developed for conducting measurements of PhotoBiology, including photosynthesis. It consists of the PB Light Source and electronic components which are an integral part of the NextGen-O2k. Measurements are recorded and evaluated with the DatLab 8 software. | |
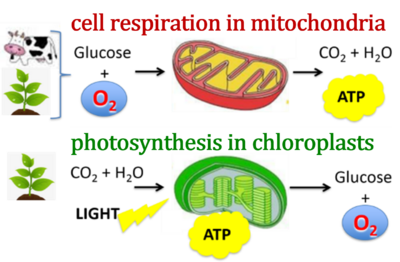
| PhotoBiology | PB | PhotoBiology is the science of the effect of light on biological processes. This includes photosynthesis, photochemistry, photophysics, photomorphogenesis, vision, bioluminescence, circadian rhythms and photodynamic therapy. Phototoxicity results from non-ionizing radiation (i.e. ultraviolet, visible and infrared radiation). Non-ionizing radiation is any type of electromagnetic radiation that does not carry enough energy per quantum (photon energy below 10 eV) to completely remove an electron from an atom or molecule. When photons interact with molecules, the molecules can absorb the photon energy and become excited, reacting with surrounding molecules and stimulating "photochemical" and "photophysical" changes. Respiration may be affected by light during photosynthesis or in dark respiration, with the transient response of light-enhanced dark respiration. |
| Photodecomposition | PD | Photodecomposition or photodegradation is the process of decay of organic material induced by increasing light intensity. Under aerobic conditions, the enhancement of photodecomposition by light intensity can be quantified by oxygen consumption in a controlled light regime. |
| Photosynthesis | PS | Photosynthesis is the process that converts light energy into chemical energy which is subsequently transformed to the physiological energy demand. Photosynthesis has a light-dependent and light-independent (dark) phase. In plants, algae, and cynobacteria, light energy is absorbed during the light phase by the pigment chlorophyll and used to split water and generate adenosine triphosphate (ATP) and reducing power - nicotinamide adenine dinucleotide phosphate (NADPH), with the net production of O2 as a waste product. During the dark phase ATP and NADPH are used to synthesize carbohydrates from CO2 through the metabolic pathway called Calvin-Benson cycle. Oxygenic photosynthesis is responsible for producing and maintaining the oxygen concentration of the Earth’s atmosphere. In bacteria such as cyanobacteria, photosynthesis involves the plasma membrane and the cytoplasm. In eukaryotic cells (plants and algae), photosynthesis takes place in the chloroplasts. |
| Q-Module | Q-Module | The Q-Module, developed for measuring the Q redox state and cyclic voltammetry, is supported by the NextGen-O2k and consists of the Q-Sensor, integrated electronic components in the O2k, and the DatLab software. |
| Q-Sensor | The Q-Sensor has been designed as a part of the Q-Module for measurements with cyclic voltammetry and voltammetry, allowing for analysis of the Q redox state. The Q-Stopper with the reference electrode is called Q-Sensor, which is plugged in the NextGen-O2k. A three-electrode system is used to detect the Q redox state. Two of the three electrodes (glassy carbon and platinum electrode) are built into the Q-Stopper, while the reference electrode is removable (Reference-Electrode\2.4 mm). |
- Bioblast links: Q - >>>>>>> - Click on [Expand] or [Collapse] - >>>>>>>
- Coenzyme Q
- » Coenzyme Q
- » Quinone, Ubiquinone Q; oxidized
- » Quinol, Ubiquinol QH2; reduced
- » Semiquinone
- » Coenzyme Q2
- » Q-redox state
- » Q-pools
- Coenzyme Q
- Mitochondrial pathways, respiratory Complexes, and Q
- » Q-cycle
- » Q-junction
- » Convergent electron flow
- » NS-pathway
- » FNS
- » FNSGp
- Mitochondrial pathways, respiratory Complexes, and Q
- NextGen-O2k and Q-Module
- Bioblast links: NADH - >>>>>>> - Click on [Expand] or [Collapse] - >>>>>>>
Mitochondrial NADH and respiration
Mitochondrial NADH production
NextGen-O2k
- Bioblast links: Hydrogen peroxide - >>>>>>> - Click on [Expand] or [Collapse] - >>>>>>>
- Specific
- General
- Bioblast links: ATP production - >>>>>>> - Click on [Expand] or [Collapse] - >>>>>>>
Phosphorylation pathway
- Phosphorylation pathway substrates
- » ADP
- » ATP
- » Inorganic phosphate
- Phosphorylation pathway substrates
- Phosphorylation pathway inhibitors
Coupling control
Respiratory complexes and coupling
ATP production measurement
- Bioblast links: pH and protons - >>>>>>> - Click on [Expand] or [Collapse] - >>>>>>>
- pH and protons
- » pH
- » hydrogen ion H+
- » hydron H+
- » hydronium ion H3O+
- » hydride H-
- » proton p+
- » pH buffering capacity
- » proton flux
- » proton pump versus hydrogen ion pump
- » proton leak
- » proton slip
- » protonmotive force
- pH and protons
- O2k-pH
- » O2k-Catalogue: O2k-pH ISE-Module
- » O2k-Manual pH electrode: MiPNet23.15 O2k-pH ISE-Module
- » O2k-SOP: MiPNet08.16 pH calibration
- » File:PH-Calibration-List.xls
- » NextGen-O2k, ratiometric: Carboxy SNARF 1
- » NextGen-O2k, ratiometric: HPTS
- » pH calibration buffers
- O2k-pH
- O2k-Publications
- HRFR - general
- » O2k-Manual: MiPNet22.11 O2k-FluoRespirometer manual
- » O2k signals and output
- » O2k-SOP: MiPNet14.06 Instrumental O2 background
- » MiPNet19.18A O2k-Series G: Start
- » ESD
- » O2k configuration
- » O2k control
- » O2k-FluoRespirometer
- » O2k-Main Unit#O2k-Series
- » Titration-Injection microPump
- » Compare: O2k-TPP+_ISE-Module
- HRFR - general
- DatLab
- Bioblast links: Calcium - >>>>>>> - Click on [Expand] or [Collapse] - >>>>>>>
- Inhibitors
- General
- Bioblast links: PhotoBiology and plant physiology - >>>>>>> - Click on [Expand] or [Collapse] - >>>>>>>
PhotoBiology: photosynthesis
Plant physiology: respiration
NextGen-O2k and PB-Module
- » NextGen-O2k
- » PB-Module
- » PB-Sensor
Labels:
MitoPedia:NextGen-O2k