Sommer 2020 Sci Adv
| Sommer N, Alebrahimdehkordi N, Pak O, Knoepp F, Strielkov I, Scheibe S, Dufour E, Andjelekovic A, Sydykov A, Saraji A, Petrovic A, Quanz K, Hecker M, Kumar M, Wahl J, Kraut S, Seeger W, Schermuly RT, Ghofrani HA, Ramser K, Braun T, Jacobs HT, Weissmann N, Szibor M (2020) Bypassing mitochondrial Complex III using alternative oxidase inhibits acute pulmonary oxygen sensing. Sci Adv 6:eaba0694. |
Sommer Natascha, Alebrahimdehkordi Nasim, Pak Oleg, Knoepp Fenja, Strielkov Ievgen, Scheibe Susan, Dufour Eric, Andjelkovic Ana, Sydykov Akylbek, Saraji Alireza, Petrovic Aleksandar, Quanz Karin, Hecker Matthias, Kumar Manish, Wahl Joel, Kraut Simone, Seeger Werner, Schermuly Ralph T, Ghofrani Hossein A, Ramser Kerstin, Braun Thomas, Jacobs Howard T, Weissmann Norbert, Szibor Marten (2020) Sci Adv
Abstract: Mitochondria play an important role in sensing both acute and chronic hypoxia in the pulmonary vasculature, but their primary oxygen-sensing mechanism and contribution to stabilization of the hypoxia-inducible factor (HIF) remains elusive. Alteration of the mitochondrial electron flux and increased superoxide release from complex III has been proposed as an essential trigger for hypoxic pulmonary vasoconstriction (HPV). We used mice expressing a tunicate alternative oxidase, AOX, which maintains electron flux when respiratory complexes III and/or IV are inhibited. Respiratory restoration by AOX prevented acute HPV and hypoxic responses of pulmonary arterial smooth muscle cells (PASMC), acute hypoxia-induced redox changes of NADH and cytochrome c, and superoxide production. In contrast, AOX did not affect the development of chronic hypoxia-induced pulmonary hypertension and HIF-1α stabilization. These results indicate that distal inhibition of the mitochondrial electron transport chain in PASMC is an essential initial step for acute but not chronic oxygen sensing.
• Bioblast editor: Plangger M • O2k-Network Lab: DE Giessen Weissmann N, FI Tampere Dufour E, FI Helsinki Jacobs HT, DE Jena Szibor M
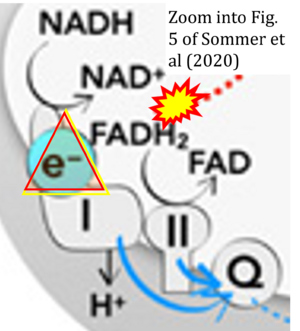
Correction: FADH2 and Complex II
- FADH2 is shown as the substrate feeding electrons into Complex II (CII). This is wrong and requires correction - for details see Gnaiger (2024).
- Gnaiger E (2024) Complex II ambiguities ― FADH2 in the electron transfer system. J Biol Chem 300:105470. https://doi.org/10.1016/j.jbc.2023.105470 - »Bioblast link«
Hydrogen ion ambiguities in the electron transfer system
Communicated by Gnaiger E (2023-10-08) last update 2023-11-10
- Electron (e-) transfer linked to hydrogen ion (hydron; H+) transfer is a fundamental concept in the field of bioenergetics, critical for understanding redox-coupled energy transformations.
- However, the current literature contains inconsistencies regarding H+ formation on the negative side of bioenergetic membranes, such as the matrix side of the mitochondrial inner membrane, when NADH is oxidized during oxidative phosphorylation (OXPHOS). Ambiguities arise when examining the oxidation of NADH by respiratory Complex I or succinate by Complex II.
- Oxidation of NADH or succinate involves a two-electron transfer of 2{H++e-} to FMN or FAD, respectively. Figures indicating a single electron e- transferred from NADH or succinate lack accuracy.
- The oxidized NAD+ is distinguished from NAD indicating nicotinamide adenine dinucleotide independent of oxidation state.
- NADH + H+ → NAD+ +2{H++e-} is the oxidation half-reaction in this H+-linked electron transfer represented as 2{H++e-} (Gnaiger 2023). Putative H+ formation shown as NADH → NAD+ + H+ conflicts with chemiosmotic coupling stoichiometries between H+ translocation across the coupling membrane and electron transfer to oxygen. Ensuring clarity in this complex field is imperative to tackle the apparent ambiguity crisis and prevent confusion, particularly in light of the increasing number of interdisciplinary publications on bioenergetics concerning diagnostic and clinical applications of OXPHOS analysis.
Labels: MiParea: Respiration
Stress:Hypoxia Organism: Rat Tissue;cell: Other cell lines Preparation: Intact cells Enzyme: Complex II;succinate dehydrogenase
HRR: Oxygraph-2k
2020-04, AOX