Talk:Digitonin
Anna Pakula
Dear MiPNet-members,we work with C2C12 cells and 3T3 cells on Oxygraph Oroboros 2k. The amount of cells is: 1 million cells in 2ml respiration buffer: MIRO5 and/or medium D (Hepes 200mM, KH2PO4 125mM, MgCl2 100mM, sucrose 2,5M according to protocol Yan Lab by Ying (Lynn) Li, 07.12.2013). Cells are trypsinized with 0,05% trypsin-EDTA and centrifuged (800rpm, 3 min). After counting the cells and trypan blue test ( ~95% cells were alive), we added into the pellet respiration buffer with 3ul digitonin ( for 1 mln cells, stock 10mg/ml) and put it into chambers. We increased gradually digitonin concentrations to 9ul or even more, without seeing any differences. The O2 concentration max reached ~180 [mmol/ml] at the start of experiment. The problem (for both C2C12 and 3T3 cells) we have is following: after addition of substrates (concentrations and order according to Kuznetsov A et al, 2008 protocol) we did not see any kind of changes in the respiration rate at all. After taking out the cells from chambers their permeabilization was checked by Trypan blue staining and cells were positive stained after the experiment. After many tests we think there is a problem with digitonin and think to replace it with saponin. If somebody used to work with both of them what concentration do you think will be worth to start experiment with? We did not wash cells to remove digitonin after the permeabilization as it was suggested by Fischer-Wellman KH, et al. 2013) and we are not sure if it could have such a big meaning for our cells? What do you think might be the major problem that our experiments do not work at all? We will be thankful for any suggestions from your side. Thank you in advance
Alexandra Latini
Your chambers are not contaminated with inhibitors, right? It happened to me many times, even when they were exhaustively cleaned up.
Bernd Schoepf
I am using Digitonin to permeabilize my PCa & BCa cell lines (PC3, LNCaP, RWPE1 and some more), but I never had any problems so far.... Could you maybe provide or post a trace so that we have more details? And if you maybe post the exact SUIT and washing procedures after your experiments we could also elaborate on that (e.g. saturating concentrations, inhibitor carry overs etc). What concentration/ammount did you use (microgramms per 10^6 cells)? Which stock solution did you use? Note that it will take some time for Digitonin to fully permeabilize the membrane! You can see it nicely when starting from (15 min of) Routine state (R). You should reach Leak state (L) within some other 10 to 15 minutes. Did you perform a titration experiment (adding Dig stepwise after substrates + ADP)? If not, I can mail you one of my traces and the SUIT. I suggest you run your rxperiments under normoxic conditions?
Alessandro Pontoglio
I worked on HepG2 cells with and without permeabilization. I read about your troubles and if you agree I'd like to ask you for some informations : 1-Your cells have been treated with digitonin 3ul reaching 9ul, was this final volume deduced by a titration with digitonin and a substrate or from a protocol already existing? 2-The protocol from Kuznetsov is....... (I can't access articles from this location) 3-Have you already managed the protocol above using only 3ul of digitonin? When I performed respiration tests on HepG2 cells, I used the same ratios chamber volume:# of cells:digitonin volume=2ml:1000000cells:3ul digitonin you are experiencing. I also checked for their permeabilization after respiration experiments (Trypan Blue) and found that they were permeabilized. At now, what I can suggest you (even if I couldn't access that protocol) : -put cells into the medium (MiR05); -pour them into the oxygraph chamber; -add the first unpermeant substrates (pyruvate or glutamate and malate); -add only 3ul of digitonin just to check for a decrease in respiration from the value initially highlighted (my HepG2 from 25pmoles/(sec*10^6cells) to 10pmoles/(sec*10^6cells) after addition of glutamate, malate and finally only 3ul digitonin). If not decreased respiration values are observed try adding more digitonin (...). I really hope you can solve your troubles with those cells. Let me know if you overcome this situation.
Stephanie Wohlgemut
We were having problems with digitonin on and off for a while. The cleaning procedure after a SUIT protocol turned out to be crucial to remove inhibitors before the next experimental run (see Oroboros recommendations for cleaning, which worked well for us). Another issue was the digitonin concentration. We ended up titrating from very low to very high concentrations (below to way above the recommended amount), and it turned out that the best concentration for our cells was indeed the recommended 10ug/Mio cells (= 1 uL of a stock of 10mg/mL EtOH; see MiPNet 09.12). Have you tried to go lower than the 3 uL/Mio cells = 30 ug/Mio cells? I hope that you will resolve the issue. Please keep the group updated, as this seems to be an ongoing and frequent issue. Best, Stephanie.
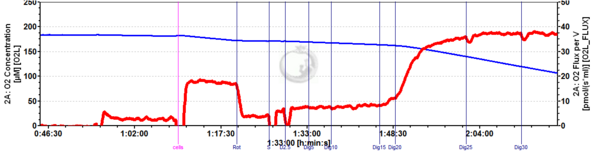
Examples for optimization of digitonin for permeabilizing cells
Permeabilization of C2C12 mouse myoblast cells with digitonin
C2C12 mouse myoblast cells (0.5 million per ml)
Experimental buffer: MiR05
Rotenone: 1 µg/ml; Succinate: 10 mM; ADP: 2.5 mM; Digitonin: 5 mg/ml stock added in 1 µl steps up to 10 µg/ml
Experimental conditions: 2 ml chamber volume, T = 37.0 °C
Figure legends: Digitonin concentration for permeabilization of cells must be optimized for each cell type/line and cell concentration used in respirometry. To this end, cells at the desired concentrations are introduced into the respiration chamber and the protocol shown here is applied. First, the ROUTINE rate of respiration observed in intact cells is determined. In this condition cells respire fuelled by endogenous substrates, since the respiration medium MiR05 does not contain any external substrates. Upon addition of complex I inhibitor rotenone respiration is blocked. Subsequently added substrates succinate and ADP do not stimulate respiration of intact cells, since there is no or only very limited uptake by the cells. The small increase of respiration may thus reflect the presence of a small percentage of damaged/dead cells. In the following, digitonin is added in small steps to progressively permeabilize the plasma membrane up to an optimum allowing unlimited access of substrates to the mitochondria, while leaving the mitochondrial membranes intact. Overtitration thus must be avoided as this will damage mitochondrial membranes and in consequence reduce respiration.
Permeabilization of MG-63 human bone osteosarcoma cells with digitonin
MG-63 human bone osteosarcoma cells (0.3 mio/ml)
Experimental buffer: MiR05
Rotenone: 1 µg/ml; Succinate: 10 mM; ADP: 2.5 mM; Digitonin: 5 mg/ml stock added in 1 and 2 µl steps up to 30 µg/ml
Experimental conditions: 2 ml chamber volume, T = 37.0 °C
Figure legends: Optimization of digitonin concentration for permeabilization of MG-63 cells, following the protocol described above for C2C12 cells.
Popular Bioblast page
Digitonin has been accessed more than
- 10,000 times (2018-10-18)
- 5,000 times (2015-09-16)