Difference between revisions of "Count"
| Line 1: | Line 1: | ||
{{MitoPedia | {{MitoPedia | ||
|abbr=''N<sub>X</sub>'' [x] | |abbr=''N<sub>X</sub>'' [x] | ||
|description=The '''number of entities''', ''N<sub>X</sub>'', is different from a pure number, ''N''. Whereas a pure number does not have a unit, a number of entities is composed of the numerical value and the countable entity, with the unit of a count, unit | |description=The '''number of entities''', ''N<sub>X</sub>'', is different from a pure number, ''N''. Whereas a pure number does not have a unit, a number of entities is composed of the numerical value and the countable entity, with the unit of a count, counting unit [x]. 'Number of entities' is synonymous with 'count'. Unfortunately, the counting unit [x] is not explicitly considered by the SI and IUPAC (Mohr and Philipps 2015). This causes confusion, since then the unit 'joule' [J] relates without discrimination to both: (''1'') exergy of the system (instrumental chamber) or the (sub)sample in the system, and (2) exergy per countable entity (cells, particles, molecules, ions, electrons, countable objects). In contrast, joule per counting unit [J∙x<sup>-1</sup>] clearly indicates exergy per entity. The unit [x] is a [[motive unit]]. The name 'counting unit' is proposed for the unit [x]. x ('times') indicates how many 'times' different members of the defined entity re-occur in the system — not how many times the same member of the defined entity is re-counted. If appropriate, it is convenient to write simply 'unit' instead of 'counting unit'. Examples: [[body mass]] expressed in 'kilograms per unit' [kg·x<sup>-1</sup>]; the [[Avogadro constant]], ''N''<sub>A</sub>, expressed in 'counting units per mole' [x·mol<sup>-1</sup>]; recurring events expressed in 'units per second' (times per second) [x·s<sup>-1</sup>]. | ||
|info=[[Gnaiger 2020 MitoPathways]] | |info=[[Gnaiger 2020 MitoPathways]] | ||
}} | }} | ||
Revision as of 23:09, 14 May 2020
Description
The number of entities, NX, is different from a pure number, N. Whereas a pure number does not have a unit, a number of entities is composed of the numerical value and the countable entity, with the unit of a count, counting unit [x]. 'Number of entities' is synonymous with 'count'. Unfortunately, the counting unit [x] is not explicitly considered by the SI and IUPAC (Mohr and Philipps 2015). This causes confusion, since then the unit 'joule' [J] relates without discrimination to both: (1) exergy of the system (instrumental chamber) or the (sub)sample in the system, and (2) exergy per countable entity (cells, particles, molecules, ions, electrons, countable objects). In contrast, joule per counting unit [J∙x-1] clearly indicates exergy per entity. The unit [x] is a motive unit. The name 'counting unit' is proposed for the unit [x]. x ('times') indicates how many 'times' different members of the defined entity re-occur in the system — not how many times the same member of the defined entity is re-counted. If appropriate, it is convenient to write simply 'unit' instead of 'counting unit'. Examples: body mass expressed in 'kilograms per unit' [kg·x-1]; the Avogadro constant, NA, expressed in 'counting units per mole' [x·mol-1]; recurring events expressed in 'units per second' (times per second) [x·s-1].
Abbreviation: NX [x]
Reference: Gnaiger 2020 MitoPathways
Communicated by Gnaiger Erich 2019-08-15, last update 2020-05-14
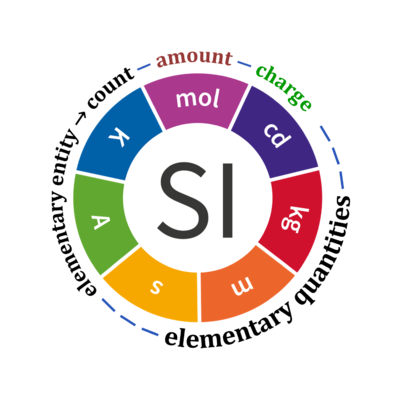
Base quantities and number of entities
Quantity Symbol for quantity Q Symbol for dimension Name of abstract unit uQ Symbol for unit uQ [*] elementary entity *,$ UX U elementary unit x count *,$ NX = N·UX X elementary unit x amount of substance *,§ nX = NX·NA-1 N mole mol charge *,€ Qel = zX·e·NX I·T coulomb C = A·s length l L meter m mass m M kilogram kg time t T second s electric current I I ampere A thermodynamic temperature T Θ kelvin K luminous intensity Iv J candela cd
- [*] SI units, except for the canonical 'elementary unit' [x]. The following footnotes are canonical comments, related to iconic symbols.
- * For the elementary quantities NX, nX, and Qel, the entity-type X of the elementary entity UX has to be specified in the text and indicated by a subscript: nO2; Nce; Qel.
- $ Count NX equals the number of elementary entities UX. In the SI, the quantity 'count' is explicitly considered as an exception: "Each of the seven base quantities used in the SI is regarded as having its own dimension. .. All other quantities, with the exception of counts, are derived quantities" (Bureau International des Poids et Mesures 2019 The International System of Units (SI)). An elementary entity UX is a material unit, it is not a count (UX is not a number of UX). NX has the dimension X of a count and UX has the dimension U of an elementary entity; both quantities have the same abstract unit, the 'elementary unit' [x].
- § Amount nX is an elementary quantity, converting the elementary unit [x] into the SI base unit mole [mol] using the Avogadro constant NA.
- € Charge is a derived SI quantity. Charge is an elementary quantity, converting the elementary unit [x] into coulombs [C] using the elementary charge e, or converting moles [mol] into coulombs [C] using the Faraday constant F. zX is the charge number per elementary entity UX, which is a constant for any defined elementary entity UX. Qel = zX·F·nX
Stating quantity values being pure numbers (p. 151)
- There are also some quantities that cannot be described in terms of the seven base quantities of the SI, but have the nature of a count. Examples are a number of molecules, a number of cellular or biomolecular entities (for example copies of a particular nucleic acid sequence), or degeneracy in quantum mechanics. Counting quantities are also quantities with the associated unit one. The unit one is the neutral element of any system of units – necessary and present automatically. There is no requirement to introduce it formally by decision. Therefore, a formal traceability to the SI can be established through appropriate, validated measurement procedures (Section 2.3.3, p. 136).
- As discussed in Section 2.3.3, values of quantities with unit one, are expressed simply as numbers. The unit symbol 1 or unit name “one” are not explicitly shown. SI prefix symbols can neither be attached to the symbol 1 nor to the name “one”, therefore powers of 10 are used to express particularly large or small values.
- Quantities that are ratios of quantities of the same kind (for example length ratios and amount fractions) have the option of being expressed with units (m/m, mol/mol) to aid the understanding of the quantity being expressed and also allow the use of SI prefixes, if this is desirable (μm/m, nmol/mol). Quantities relating to counting do not have this option, they are just numbers.
- The internationally recognized symbol % (percent) may be used with the SI. When it is used, a space separates the number and the symbol %. The symbol % should be used rather than the name “percent”. In written text, however, the symbol % generally takes the meaning of “parts per hundred”. Phrases such as “percentage by mass”, “percentage by volume”, or “percentage by amount of substance” shall not be used; the extra information on the quantity should instead be conveyed in the description and symbol for the quantity.
- The term “ppm”, meaning 10-6 relative value, or 1 part in 106, or parts per million, is also used. This is analogous to the meaning of percent as parts per hundred. The terms “parts per billion” and “parts per trillion” and their respective abbreviations “ppb” and “ppt”, are also used, but their meanings are language dependent. For this reason the abbreviations ppb and ppt should be avoided.
- Reference: Bureau International des Poids et Mesures (2019) The International System of Units (SI). 9th edition:117-216 ISBN 978-92-822-2272-0. - »Open Access pdf«
References
- Bureau International des Poids et Mesures (2019) The International System of Units (SI). 9th edition:117-216 ISBN 978-92-822-2272-0. - »Open Access pdf«
- Gnaiger E (2019) Mitochondrial pathways and respiratory control. An introduction to OXPHOS analysis. 5th ed. Mitochondr Physiol Network 24.05. Oroboros MiPNet Publications, Innsbruck:112 pp. - »Bioblast link«
- Mohr PJ, Phillips WD (2015) Dimensionless units in the SI. Metrologia 52:40-7. - »Bioblast link«
- Bioblast links: SI base units - >>>>>>> - Click on [Expand] or [Collapse] - >>>>>>>
- Entity, count, and number, and SI base quantities / SI base units
Quantity name Symbol Unit name Symbol Comment elementary UX elementary unit [x] UX, UB; [x] not in SI count NX elementary unit [x] NX, NB; [x] not in SI number N - dimensionless = NX·UX-1 amount of substance nB mole [mol] nX, nB electric current I ampere [A] A = C·s-1 time t second [s] length l meter [m] SI: metre mass m kilogram [kg] thermodynamic temperature T kelvin [K] luminous intensity IV candela [cd]
- Fundamental relationships
- » Avogadro constant NA
- » Boltzmann constant k
- » elementary charge e
- » Faraday constant F
- » gas constant R
- » electrochemical constant f
- Fundamental relationships
- SI and related concepts
MitoPedia concepts:
Ergodynamics