Difference between revisions of "Cytochrome c control efficiency"
Beno Marija (talk | contribs) |
|||
| Line 18: | Line 18: | ||
}} | }} | ||
__TOC__ | __TOC__ | ||
:::: The outer mitochondrial membrane integrity can be evaluated by stimulation of respiration by exogenously added cytochrome ''c'' and expressing the normalized c-effect as the [[flux control factor]]<ref> Gnaiger E. Flux control factor: normalization of mitochondrial respiration. Mitochondr Physiol Network. »[[Flux control factor]]</ref>, ''FCF''<sub>c</sub>. | :::: The outer mitochondrial membrane integrity can be evaluated by stimulation of respiration by exogenously added cytochrome ''c'' and expressing the normalized c-effect as the [[flux control factor]]<ref> Gnaiger E. Flux control factor: normalization of mitochondrial respiration. Mitochondr Physiol Network. »[[Flux control factor]]</ref>, ''FCF''<sub>c</sub>. | ||
Revision as of 15:58, 9 July 2020
- high-resolution terminology - matching measurements at high-resolution
Cytochrome c control efficiency
Description
The cytochrome c control factor expresses the control of respiration by externally added cytochrome c, c, as a fractional change of flux from substrate state CHO to CHOc. In this flux control factor (FCFc), CHOc is the reference state with stimulated flux; CHO is the background state with CHO substrates, upon which c is added,
FCFc = (JCHOc-JCHO)/JCHOc.
Abbreviation: FCFc
Reference: Gnaiger 2013 Abstract MiP2013, Laner 2014 Abstract MiP2014
MitoPedia concepts:
Respiratory control ratio
MitoPedia methods:
Respirometry
MitoPedia topics: Substrate and metabolite
- The outer mitochondrial membrane integrity can be evaluated by stimulation of respiration by exogenously added cytochrome c and expressing the normalized c-effect as the flux control factor[1], FCFc.
Determination of cytochrome c loss
- Addition of cytochrome c (10 µM final concentration)[2] may stimulate OXPHOS capacity or ET capacity and thus provides evidence for the occurrence of cytochrome c loss.
Cytochrome c test
- When using cytochrome c as quality control for permeabilized muscles from various species, which cytochrome c should we use (a wide range of types of cytochrome c is available from Sigma-Aldrich) and at which concentration?
- We apply routinely cytochrome c from Sigma C 7752.[3] It would be interesting to compare the consequence of the application of different sources of cytochrome c.
- For more information, see: [[Cytochrome_c#Application_in_HRR:_storage_and_stock_solution_of_c]|Application in HRR: storage and stock solution of cytochrome c]
- In a detailed discussion on the dependence of respiration in isolated mitochondria and permeabilized cardiac fibers, we showed for the first time that cytochrome c kinetics is different when studying a segment of the Electron transfer pathway (CII-linked: succinate+rotenone) versus the isolated step of cytochrome c oxidase (CIV; ascorbate+TMPD+antimycin A). For CII-linked respiration, an exernal cytochrome c concentration of 10 µM yields kinetic saturation (monophasic hyperbolic), but kinetics is biphasic for CIV and 10 µM is not saturating. [4],[2]
- Importantly, cytochrome c increases the chemical background oxygen flux in the presence of ascorbate and TMPD, dependent on oxygen concentration and cytochrome c concentration, and appropriate chemical background corrections are required. [5],[6] Without ascorbate and TMPD, added cytochrome c is stable.
- Evaluation of the cytochrome c effect, when respiration is slightly unstable: Mark respiration just before cytochrome c addition and after. Take these to values to calculate the increase of respiration due to cytochrome c addition.
At which step of the protocol should cytochrome c be added?
- If a stimulatory effect of cytochrome c is observed, respiratory capacities measured before cytochrome c addition might be cytochrome c-limited and therefore underestimated. This provides an argument to add cytochrome c at an early stage of the protocol.[7]
- It is important not to add cytochrome c in a LEAK state: There is always an unexplained activation of respiration, unrelated to the injury of the outer mt-membrane. Add cytochrome c only after activation by ADP.
Cytochrome c release
Cytochrome c release induced by sample preparation
- The procedures required for mitochondrial preparations (homogenization, isolation, permeabilization of the plasma membrane with chemicals, etc) are key steps that determine the quality of the mitochondrial respiration assessments. During the isolation of the organelles or the permeabilization of the cells/fibers, one must ensure the integrity of the mitochondrial outer membrane. However, some of the techniques used, like the disruption of the tissue for mitochondria isolation or the permeabilization of the cells' plasma membrane with digitonin, might compromise its integrity. For this reason, the addition of cytochrome c is routinely used to test the outer membrane permeability and to evaluate the quality of the mitochondrial preparation. Preparation-induced damage of the outer mitochondrial membrane, with subsequent loss of cytochrome c, will be detected by stimulation of respiration after the addition of cytochrome c. It is important to note that preparation-induced damage can also affect the respiratory complexes. Therefore, experimental runs showing a relevant preparation-induced cytochrome c release should be excluded from the final data set.
- It is expected to find stimulation in the respiration rate when cytochrome c is added, but the percentage of increase should be determined empirically because it is sample-specific. For example, in the case of HEK 293 cells permeabilized with digitonin, it is well characterized that the cytochrome c has a minimum stimulatory effect in the OXPHOS respiration. However, in other preparations such as isolated mitochondria from mouse heart, a significant increase in respiration has been described. In perfectly prepared muscle fibers cytochrome c should have no stimulatory effect on maximum respiratory activity, in liver biopsies a small effect is observed, even in carefully prepared samples. If there are no data available in the literature for specific sample preparations, it is necessary to accumulate enough data and look for consistency. It is then possible to determine a threshold of the percentage increase to decide if a mitochondrial preparation has good quality and outer membrane integrity.
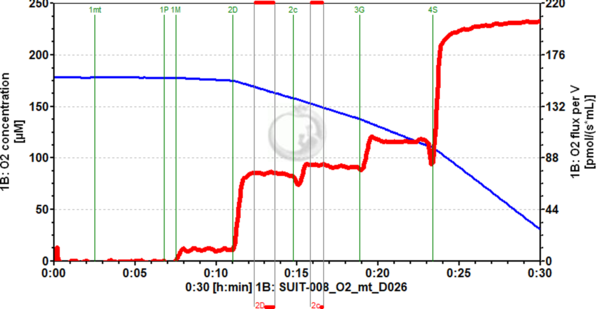
Figure 1. Example of cytochrome c addition (2c) in OXPHOS state, using pyruvate and malate as substrates, with mitochondria isolated from mouse heart (following the same preparation described for rat heart MiPNet20.15 IsolationRatHeart-mt). An increase in O2 flux (red trace, right axis) can be seen upon cytochrome c addition, which has been consistently seen for this kind of mitochondrial preparation. Image from SUIT-008_O2_mt_D026.DLP (experiment 2019-02-19 P1-02), DatLab 7.4.
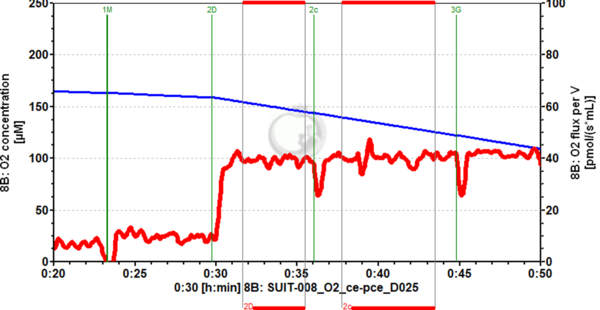
Figure 2. Example of cytochrome c addition (2c) in OXPHOS state, using pyruvate and malate as substrates, with cryopreserved HEK-293 cells, permeabilized with digitonin in the O2k chamber. Hardly any increase in O2 flux (red trace, right axis) can be seen upon cytochrome c addition, which has been consistently seen for this kind of mitochondrial preparation. Image from SUIT-008_O2_ce-pce_D025.DLP (experiment 2017-02-08 P1-02), DatLab 7.4.
Cytochrome c release induced by treatment
- Treatment-triggered cytochrome c release, e.g. cell death induction, has to be distinguished from preparation induced damage. If cytochrome c is released as a result of apoptosis induction, this is a biological phenomenon and a relevant result.
Interpretation of the cytochrome c control factor
- Cytochrome c release throughout the mitochondrial population in a tissue in vivo may induce a pathophysiological limitation of respiratory capacity, since the released cytochrome c accumulates in the cytosol at a low (non-saturating) concentration. If all mitochondria in a particular cell release their cytochrome c, then the cytosolic concentration increases to a level nearly saturating respiration [8]. If the pathology involves a change of mt-membrane properties which render the mitochondria more vulnerable to experimental damage during tissue preparation, then the cytochrome c effect is to be interpreted differently. Such a pathophysiological modification is specifically revealed by the respirometric approach to OXPHOS analysis with SUIT protocols.
References
- ↑ Gnaiger E. Flux control factor: normalization of mitochondrial respiration. Mitochondr Physiol Network. »Flux control factor
- ↑ 2.0 2.1 Kuznetsov AV, Schneeberger S, Seiler R, Brandacher G, Mark W, Steurer W, Saks V, Usson Y, Margreiter R, Gnaiger E (2004) Mitochondrial defects and heterogeneous cytochrome c release after cardiac cold ischemia and reperfusion. Am J Physiol Heart Circ Physiol 286: H1633–H1641. »Open Access
- ↑ Fontana-Ayoub M, Fasching M, Gnaiger E (2014) Selected media and chemicals for respirometry with mitochondrial preparations. Mitochondr Physiol Network 03.02(17):1-9. »Bioblast link«
- ↑ Gnaiger E, Kuznetsov AV (2002) Mitochondrial respiration at low levels of oxygen and cytochrome c. Biochem Soc Trans 30: 242-248. »Bioblast link«
- ↑ Renner K, Amberger A, Konwalinka G, Kofler R, Gnaiger E (2003) Changes of mitochondrial respiration, mitochondrial content and cell size after induction of apoptosis in leukemia cells. Biochim Biophys Acta 1642: 115-123. »PMID: 12972300
- ↑ Kuznetsov AV, Gnaiger E (2010) Oxygraph assay of cytochrome c oxidase activity: chemical background correction. Mitochondr Physiol Network 06.06(07): 1-4. »Bioblast link«
- ↑ Laner V, Boushel RC, Hamilton KL, Miller BF, Williamson KK, Davis MS, Gnaiger E (2014) Cytochrome c flux control factor as a quality criterion in respiratory OXPHOS analysis in canine permeabilized fibres. Mitochondr Physiol Network 19.13. »Bioblast link«
- ↑ Brown GC, Borutaite V (1999) Nitric oxide, cytochrome c and mitochondria. Biochem. Soc. Symp. 66, 17–25. »PMID: 10989653