Difference between revisions of "DTPA"
From Bioblast
| (37 intermediate revisions by 6 users not shown) | |||
| Line 1: | Line 1: | ||
{{MitoPedia | {{MitoPedia | ||
|abbr=DTPA | |abbr=DTPA | ||
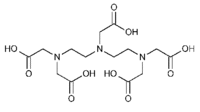
|description='''DTPA''' ( | |description='''DTPA''' (Diethylenetriamine-N,N,N',N,N-pentaacetic acid, pentetic acid,(Carboxymethyl)imino]bis(ethylenenitrilo)-tetra-acetic acid) is a polyaminopolycarboxylic acid (like EDTA) chelator of metal cations. DTPA wraps around a metal ion by forming up to eight bounds, because each COO- group and and N-center serves a center for chelation. With transition metals the number of bounds is less than eight. The compound is not cell membrane permeable. In general, it chelates multivalent ions stronger than EDTA. | ||
}} | }} | ||
[[File:DTPA structure.gif|right|200px|DTPA structure]] | |||
__TOC__ | |||
== | == Application in [[HRR]] == | ||
:::'''Fluorometry:''' | |||
::::* In [[Amplex UltraRed]] assay systems with [[HRP]], there is a spontaneous increase in the UltoroxRed fluorescence signal over the experimental time without any biological sample. This high background flux can be related to iron contamination. We have done a set of experiments, where we added DTPA as an iron-chelator into the O2k-chamber during AmR measurements. As a matter of fact, DTPA significantly reduced the high chemical background flux in MiR05 without affecting respiration. | |||
::::* Administration of DTPA into the O2k-chamber is recommended before all other chemicals because the iron chelation of the compound is time-dependent; it takes 10-15 min. | |||
::::* Experiments may also be run without DTPA. | |||
:::'''DTPA''' (C<sub>2</sub>H<sub>2</sub>O<sub>2</sub>); Dr.Ehrenstorfer GmbH (E 13095000) or Sigma Aldrich (D-1133); CAS 67-43-6; The commercial powder can be stored at room temperature. The stock solution has to be stored at -20 °C; M: 393.35 g·mol<sup>-1</sup> | |||
::::* | ::::* '''Solubility:''' It is soluble in water (4,8 g/l at 25 °C), but not readily soluble in ethanol or apolar solvents. | ||
::::* '''Stability constant:''' see: [http://www.dojindo.com/Images/Product%20Photo/Chelate_Table_of_Stability_Constants.pdf]: | |||
:::::{| class="wikitable" | |||
::::* Stability | |- | ||
:::: | !Metal !! DTPA !! EDTA | ||
|- | |||
|Ca(II)|| 10.74 ||10.96 | |||
|- | |||
|Mg(II)|| 9.3 ||8.69 | |||
|- | |||
|Fe(II)|| 16.5 ||14.3 | |||
|- | |||
|Fe(III) || 28.6 ||25.1 | |||
|- | |||
|Mn(II) || 15.6 ||14.04 | |||
|- | |||
|Zn(II) || 18.75 || 16.5 | |||
|- | |||
|} | |||
::: '''Preparation of the 5 mM stock solution of DTPA:''' | |||
::::# Weigh 1.967 mg of DTPA in a 2 mL glass vial. | |||
::::# Dissolve in 500 µL H<sub>2</sub>O and add 6 µL of 5 M KOH to set the pH. | |||
::::# Heat up the solution up to 33 °C. | |||
::::# Add 2 µL 5 M KOH to adjust the pH ~ 8-9. | |||
::::# Add 500 µL H<sub>2</sub>O to get 5 mM stock solution. | |||
::::# Divide into 50 µL portions in Eppendorf tubes. | |||
::::# Store at -20 °C. | |||
:::»'''O2k manual titrations:''' [[MiPNet09.12 O2k-Titrations]] | |||
::::* Final concentration in O2k-chamber: 15 µM | ::::* Final concentration in O2k-chamber: 15 µM | ||
::::* Stock solution: | ::::* Stock solution: 5 mM | ||
::::* Titration volume: 6 µL | |||
{{MitoPedia methods | |||
|mitopedia method=Fluorometry | |||
}} | |||
{{MitoPedia O2k and high-resolution respirometry}} | |||
{{MitoPedia topics}} | |||
Revision as of 14:44, 22 August 2022
Description
DTPA (Diethylenetriamine-N,N,N',N,N-pentaacetic acid, pentetic acid,(Carboxymethyl)imino]bis(ethylenenitrilo)-tetra-acetic acid) is a polyaminopolycarboxylic acid (like EDTA) chelator of metal cations. DTPA wraps around a metal ion by forming up to eight bounds, because each COO- group and and N-center serves a center for chelation. With transition metals the number of bounds is less than eight. The compound is not cell membrane permeable. In general, it chelates multivalent ions stronger than EDTA.
Abbreviation: DTPA
Application in HRR
- Fluorometry:
- In Amplex UltraRed assay systems with HRP, there is a spontaneous increase in the UltoroxRed fluorescence signal over the experimental time without any biological sample. This high background flux can be related to iron contamination. We have done a set of experiments, where we added DTPA as an iron-chelator into the O2k-chamber during AmR measurements. As a matter of fact, DTPA significantly reduced the high chemical background flux in MiR05 without affecting respiration.
- Administration of DTPA into the O2k-chamber is recommended before all other chemicals because the iron chelation of the compound is time-dependent; it takes 10-15 min.
- Experiments may also be run without DTPA.
- DTPA (C2H2O2); Dr.Ehrenstorfer GmbH (E 13095000) or Sigma Aldrich (D-1133); CAS 67-43-6; The commercial powder can be stored at room temperature. The stock solution has to be stored at -20 °C; M: 393.35 g·mol-1
- Fluorometry:
- Solubility: It is soluble in water (4,8 g/l at 25 °C), but not readily soluble in ethanol or apolar solvents.
- Stability constant: see: [1]:
Metal DTPA EDTA Ca(II) 10.74 10.96 Mg(II) 9.3 8.69 Fe(II) 16.5 14.3 Fe(III) 28.6 25.1 Mn(II) 15.6 14.04 Zn(II) 18.75 16.5
- Preparation of the 5 mM stock solution of DTPA:
- Weigh 1.967 mg of DTPA in a 2 mL glass vial.
- Dissolve in 500 µL H2O and add 6 µL of 5 M KOH to set the pH.
- Heat up the solution up to 33 °C.
- Add 2 µL 5 M KOH to adjust the pH ~ 8-9.
- Add 500 µL H2O to get 5 mM stock solution.
- Divide into 50 µL portions in Eppendorf tubes.
- Store at -20 °C.
- »O2k manual titrations: MiPNet09.12 O2k-Titrations
- Final concentration in O2k-chamber: 15 µM
- Stock solution: 5 mM
- Titration volume: 6 µL
MitoPedia methods: Fluorometry