| News and Events | Working Groups | Short-Term Scientific Missions | Management Committee | Members |
COST Action CA15203 (2016-2021): MitoEAGLE
Evolution-Age-Gender-Lifestyle-Environment: mitochondrial fitness mapping
MitoEAGLE protocols, terminology, documentation
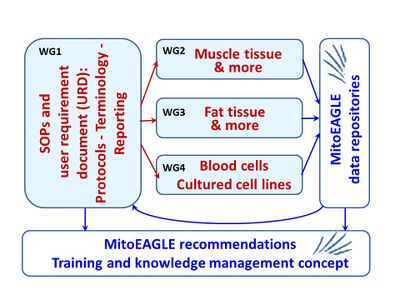
WG1
- Standard operating procedures and user requirement document: Protocols, terminology, documentation
WG1 Description
- The quality management system, scope of SOPs to be elaborated and the outline of the data repositories will be defined in a user requirement document.
Respirometric reference protocols
- A ‘library of protocols’ applied in mitochondrial respiratory physiology will be collected in a standard format, delineating the diversity of experimental approaches to assess bioenergetic function. A set of reference protocols will rigorously document (Maelstrom Research program) the state-of-the-art standards in designing, conducting, reporting, interpreting, and validating such bioenergetic tests (see also Fig. 3).
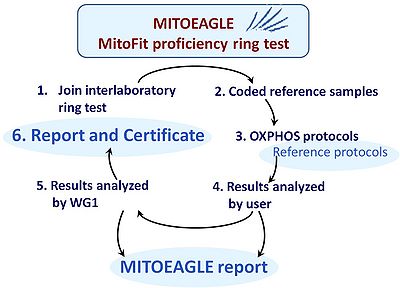
Interlaboratory proficiency test
- Inter-laboratory ring tests are a basic requirement for quality management. Such proficiency tests require a reference sample which must be homogenous, stable, representative of the diagnostic target, amenable for distribution, and economical for large-scale production. For respiratory OXPHOS analysis a reference sample of structurally and functionally intact mitochondria is not yet available to perform corresponding ring tests (in contrast to enzymatic OXPHOS ring tests). Recently, the widely applied human cell line HEK 293T was found to be potentially suitable for cryopreservation. Upon thawing the cells can be used immediately for respirometry of both intact and permeabilized cells. Thus interlaboratory proficiency testing may now be feasible as a world-wide innovation in the field of mitochondrial respiratory physiology. Participating labs may implement the test for intra- and interlaboratory validation and longitudinal performance monitoring, using a reference OXPHOS protocol (Fig. 3). The organization of the proficiency test should follow the requirements of the ISO 17043 and 13528 standards. A corresponding MITOEAGLE proficiency training shall be developed and implemented. Feedback from the participants will lead to a final adjustment of the SOPs in the proficiency test, which will be made generally available.
Instrumental platforms
- Comparison of results between and within instrumental platforms: The reference protocols and proficiency test will not define the instrumental platform, but will allow a quantitative comparison of results obtained with specific equipment available in the participating laboratories.
Nomenclature
- Harmonization of nomenclature on mitochondrial respiratory states and control parameters: The logistics of development of a database requires application of strictly defined terms for all included variables. There is no general reference available upon which a consistent terminology on mitochondrial physiology and bioenergetics can be based. The MITOEAGLE consortium, therefore, has to accomplish the ambitious goal to unify and simplify the terminology in the field for the purpose of the QMS, which will lead to the development of recommendations for the use of a common terminology in mitochondrial physiological research. A publication will be prepared as an Open Access article which will be a milestone towards a unification of concepts and nomenclature.
Knowledge management system
- With an increase in data volume and complexity comes along the requirement for standardized report formats and digital accessibility of the data. Since such a database is still missing for mitochondrial physiology, the goal of WG1 is to establish an integrated and analytic environment linking mitochondrial respiration with external data (cell physiology, anthropometry, spiroergometry, lifestyle, genomics) in the context of physical fitness evaluation and clinical diagnostics. Databases will be established combining biological and clinical data comprising proprietary and publicly available datasets into one integrated solution. Data import and export functionalities will allow data upload and mapping to corresponding objects in the database, in this way generating valuable data and allowing for project-specific as well as cross-project data mining.
- The ability to formulate meta-studies in our approach is an important success factor: The platform can aggregate the project’s complete knowledge and provides the ability to make diagnostic profiling associated with diseases. In addition to functional data, other data types such as nutritional or environmental factors associated with disease prevention can be identified and aggregated into the MITOEAGLE Knowledge Management System.
- Querying the heterogeneous data types and result representation are the most important requirements of the knowledge platform besides to data integration and modelling. The application will feature different types of visualization techniques for all integrated data types. The database will be developed using the concept of multi-tier client-server architecture. Individual components are separated corresponding to their functionality. An upstream web server handles user requests, an application server performing business logic, and a back-end database system guaranties persistent data storage. All components are additionally separated by means of network security incorporating firewalls and are hosted in a secure environment. The platform administration ensures privacy regarding proprietary data by user rights policies.
- It will be the task of WG1 to continuously collect the input from all other WGs, compare, discuss and align SOPs as far as possible, and to finally develop recommendations for quality control, data reporting and data sharing beyond the published record. In addition, WG1 will develop concepts on Open Access and institutionalized service for data management, data mining and health-care conforming standards of data interpretation. Finally, it will provide a summary on strategic dissemination and an education programme for MITOEAGLE. The education programme will ensure that not only presently participating groups will adhere to the set standards but that also groups joining in at later points may smoothly fit into the community and help to further broaden and deepen our understanding on mitochondrial physiology in health and disease.
WG1 Participants
| Country | City | Contact |
|---|---|---|
| Armenia | Yerevan | Trchounian K |
| Australia | Melbourne | Kuang Jujiao |
| Australia | Syndey | Ton Riccardo |
| Australia | Nedlands | Perks Kara L |
| Australia | Melbourne | Hewakapuge Sudinna |
| Australia | Nedlands | Filipovska Aleksandra |
| Australia | Burwood | Trewin Adam J |
| Australia | Melbourne | Lionett S |
| Australia | Clayton Victoria | Alton Lesley |
| Australia | Parkville | Hardee Justin P |
| Australia | Melbourne | Cassar Samantha |
| Australia | Melbourne | Coughlan Melinda T |
| Australia | Sydney | Hoehn Kyle L |
| Australia | Melbourne | Genders Amanda J |
| Australia | Victoria | Winwood-Smith Hugh |
| Australia | Sydney | Krycer James R |
| Australia | Queensland | Holland Olivia J |
| Australia | Melbourne | Saner Nicholas |
| Australia | Melbourne | Stepto Nigel K |
| Australia | Newtown, Sydney | Stocker Roland |
| Australia | Geelong, Victoria | McKenzie Matthew |
| Australia | Melobourne | Botella Javier |
| Australia | Southport | Vidimce Josif |
| Australia | Queensland | Dong Lan-Feng |
| Australia | Melbourne | Whitfield Jamie |
| Australia | Melbourne | Ferri Alessandra |
| Australia | Victoria | Van Bergen Nicole J |
| Australia | Queensland | Radenkovic Filip |
| Australia | Queensland | Fisher Joshua J |
| Australia | Clayton | Wolff Jonci Nikolai |
| Australia | Melbourne, VIC | Muccini Anna Maria |
| Austria | Innsbruck | Thapa Maheshwor |
| Austria | Innsbruck | Plangger Mario |
| Austria | Innsbruck | Cardoso Luiza HD |
| Austria | Innsbruck | Schartner Melanie |
| Austria | Innsbruck | Burtscher Martin |
| Austria | Innsbruck | Meszaros Andras T |
| Austria | Innsbruck | Hoppel Florian |
| Austria | Innsbruck | Di Marcello Marco |
| Austria | Innsbruck | Bergmeister Lisa |
| Austria | Innsbruck | Doerrier Carolina |
| Austria | Innsbruck | Gallee Leon |
| Austria | Innsbruck | Sobotka Ondrej |
| Austria | Innsbruck | Perez Valencia Juan Alberto |
| Austria | Innsbruck | Goebel G |
| Austria | Aldrans | Haider Markus |
| Austria | Innsbruck | Schwarzer Christoph |
| Austria | Innsbruck | Garcia-Souza Luiz F |
| Austria | Klosterneuburg | Sazanov Leonid A |
| Austria | Innsbruck | Klocker Helmut |
| Austria | Innsbruck | Laner Verena |
| Austria | Innsbruck | Nindl Veronika |
| Austria | Innsbruck | Karabatsiakis Alexander |
| Austria | Innsbruck | Schmitt Sabine |
| Austria | Innsbruck | Gnaiger Erich |
| Austria | Innsbruck | Cecatto Cristiane |
| Austria | Innsbruck | Passrugger Manuela |
| Austria | Salzburg | Kofler B |
| Austria | Innsbruck | Keller Markus A |
| Austria | Vienna | Kozlov Andrey V |
| Austria | Kapferer Werner | |
| Austria | Innsbruck | Antunes Diana |
| Belgium | Liege | Votion Dominique-Marie |
| Belgium | Namur | Arnould Thierry |
| Belgium | Leuven, Flanders | Baekelandt V |
| Belgium | Brussels | Sonveaux P |
| Belguim | Sart Tilman (Liège) | Kruse C |
| Brasil | Porto Alegre | Haas Clarissa |
| Brasilia | Limeira | Ropelle Eduardo R |
| Brazil | Rio de Janeiro | Oliveira Marcus F |
| Brazil | Santa Maria - Rio Grande do Sul | Soares Felix Alexandre Antunes |
| Brazil | Rio de Janeiro | Vieyra Adalberto |
| Brazil | Rio de Janeiro | Galina Antonio |
| Brazil | Sao Paulo | Vercesi Anibal E |
| Brazil | São Paulo | Crispim Marcell |
| Brazil | São Paulo | Kowaltowski Alicia J |
| Brazil | Sao Paulo | Silber Ariel Mariano |
| Brazil | Santa Maria - RS | Goncalves Debora Farina |
| Brazil | Jaboticabal | Oliveira Marcos Tulio |
| Canada | Charlottetown | Adiele Reginald C |
| Canada | Montréal | Breton Sophie |
| Canada | Rimouski | Blier Pierre U |
| Canada | Edmonton | Han Woo Hyun |
| Canada | Vancouver, BC | Tausan Daniel |
| Canada | Hamilton | Scott Graham R |
| Canada | Montreal, Quebec | Bergdahl Andreas |
| Canada | Quebec | Joseph Vincent |
| Canada | Ottawa | Harper Mary-Ellen |
| Canada | Moncton, NB | Pichaud Nicolas |
| Canada | Winnipeg | Chowdhury Subir Roy |
| Canada | Edmonton (Alberta) | Lemieux Helene |
| Canada | Saint John | Pulinilkunnil Thomas |
| Canada | Edmonton | Zaugg Michael |
| Canada | Rimouski | Munro Daniel |
| Canada | Antigonish | Weir M |
| Canada | Edmonton | Lucchinetti Eliana |
| Canada | Vancouver | Bovard Josh |
| Canada | Hamilton | Tarnopolsky Mark A |
| Canada | Guelph | Holloway Graham P |
| Canada | Ottawa | Darveau Charles-A |
| Chile | Coyhaique | Jana Prado Fabian |
| China | Xi`an | Liu Jiankang |
| China | Pukou, Nanjing, | Gan Zhenji |
| China | Tianjin | Zhang Yong |
| China | Fuzhou, Fujian Province | Chen Qi |
| Croatia | Zagreb | Rubelj Ivica |
| Croatia | Split | Ljubkovic M |
| Croatia | Zagreb | Saric A |
| Croatia | Zagreb | Curik I |
| Czech Republic | Hradec Kralove | Endlicher Rene |
| Czech Republic | Ceske Budejovice | Zikova Alena |
| Czech Republic | Pardubice | Handl Jiri |
| Czech Republic | Praha 2 | Danhelovska Tereza |
| Czech Republic | Hradec Kralove | Kucera Otto |
| Czech Republic | Vestec | Ezrova Zuzana |
| Czech Republic | Praha 2 | Zdrazilova Lucie |
| Czech Republic | Vestec | Davidova Eliska |
| Czech Republic | Hradec Kralove | Stankova Pavla |
| Czech Republic | Plzen -Severni predmesti | Jedlicka Jan |
| Czech Republic | Vestec | Rohlena Jakub |
| Czech Republic | Pardubice | Majtnerova Pavlina |
| Czech Republic | Hradec Kralove | Cervinkova Zuzana |
| Czech Republic | Prague 10 | Urban Tomas |
| Czech Republic | Pardubice | Rousar Tomas |
| Czech Republic | Prague | Drahota Zdenek |
| Czech Republic | Prague | Krizova J |
| Czech Republic | Prague | Houstek Josef |
| Czech rep | Hradec Kralove | Falaye T |
| Denmark | Roskilde | Dalgaard Louise T |
| Denmark | Copenhagen N | Scheibye-Knudsen Morten |
| Denmark | Copenhagen N | Mortensen OH |
| Denmark | Copenhagen | Dela Flemming |
| Denmark | Copenhagen | Nielsen B |
| Denmark | Aarhus N | Boetker Hans Erik |
| Denmark | Copenhagen | Larsen Steen |
| Denmark | Aarhus N | Jespersen Nichlas Riise |
| Denmark | Copenhagen N | Maise Chroeis K |
| Denmark | Copenhagen | Soendergaard SD |
| Denmark | Hvidovre | Nehlin Jan O |
| Denmark | Copenhagen | Gonzalez-Franquesa Alba |
| Denmark | Copenhagen | Kraunsoee R |
| Egypt | Cairo | Ali Sameh S |
| Egypt | Cairo | Abdel-Rahman Engy Ali |
| Estonia | Tallinn | Puurand Marju |
| Estonia | Tallinn | Karro N |
| Estonia | Tallinn | Laasmaa Martin |
| Estonia | Tallinn | Truu Laura |
| Estonia | Tallinn | Klepinin Aleksandr |
| Estonia | Tallinn | Kaambre Tuuli |
| Estonia | Tallinn | Klepinina Lyudmila |
| Estonia | Tallinn | Shevchuk Igor |
| Estonia | Tallinn | Tepp Kersti |
| Estonia | Tartu | Paju Kalju |
| Estonia | Tallinn | Vendelin Marko |
| Finland | Helsinki | Suomalainen Wartiovaara Anu |
| Finland | Turku | Stier Antoine |
| Finland | Helsinki | Jackson Christopher Benjamin |
| Finland | Jyvaeskylae | Kainulainen Heikki |
| France | Bordeaux cedex | Rossignol Rodrigue |
| France | Nouzilly | Collin-Chenot Anne |
| France | Martinique | Neviere Remi |
| France | Angers | Procaccio Vincent |
| France | Lille | Montaigne David |
| France | Paris | Bouillaud Frederic |
| France | Martinique | Cano Sanchez Maria Consolacion |
| France | Maisons-Alfort Cedex | Prola Alexandre |
| France | Bordeaux | Amoedo Nivea Dias |
| France | Bordeaux | Sarlak Saharnaz |
| France | Gueguen Naig | |
| France | Plouzané | Salin Karine |
| France | Tours | Dumas Jean-Francois |
| France | Paris | Armand Anne-Sophie |
| France | Angers | Spinazzi Marco |
| France | Bordeaux cedex | Mourier A |
| France | Montpellier | Wrutniak-Cabello Chantal |
| Germany | Neuherberg | Einer C |
| Germany | Ulm | Schaefer PM |
| Germany | Düsseldorf | Goy C |
| Germany | Jena | Szibor Marten |
| Germany | Biberach an der Riß | Nold V |
| Germany | Tuebingen | Kappler Lisa |
| Germany | Duesseldorf | Roden Michael |
| Germany | Frankfurt | Warnsmann Verena |
| Germany | Bremerhaven | Mark Felix Christopher |
| Germany | Giessen | Sommer Natascha |
| Germany | Frankfurt | Hamann Andrea |
| Germany | Mainz | Methner Axel |
| Germany | Gießen | Grewal Rekha |
| Germany | Wickert Anika | |
| Germany | Gießen | Silaidos Carmina |
| Germany | Duesseldorf | Jelenik T |
| Germany | Munich | Zischka Hans |
| Germany | Cologne | Pesta Dominik |
| Germany | Frankfurt am Main | Viel Christian |
| Germany | Wilhelmshaven | Salmon Pablo |
| Germany | Doermann Niklas | |
| Germany | Magdeburg | Schoenfeld Peter |
| Germany | Marburg | Vogt Sebastian |
| Germany | Cologne | Ho Dieu Hien |
| Germany | Giessen | Schulz Rainer |
| Germany | Cologne | Wiesner Rudolf J |
| Germany | Mainz | Maull Felicia |
| Germany | Duesseldorf | Piel Sarah |
| Germany | Regensburg | Renner-Sattler Kathrin |
| Germany | Freiburg | Schuele R |
| Germany | Cologne | Maciej Sarah |
| Germany | Cologne | Trifunovic Aleksandra |
| Germany | Hannover | Das Anibh Martin |
| Germany | Marburg | Ramzan Rabia |
| Germany | Rostock | Sokolova Inna |
| Germany | Duesseldorf | Haendeler Judith |
| Germany | Frankfurt | Osiewacz Heinz D |
| Greece | Thessaloniki | Lazou Antigone |
| Greece | 15771 | Andreadou Ioanna |
| Greece | Athens | Trougakos Ioannis P |
| Greece | Athens | Gumeni Sentiljana |
| Greece | Heraklion | Tavernarakis Nektarios |
| Hungary | Szeged | Boros Mihaly |
| Hungary | Budapest | Horvath G |
| Hungary | Budapest | Chinopoulos Christos |
| Hungary | Budapest | Tretter Laszlo |
| Hungary | Szeged | Ferdinandy Peter |
| Hungary | Budapest | Komlodi Timea |
| Hungary | Debrecen | Verebne Tar K |
| India | Lucknow | Gayen Jiaur |
| India | Hyderabad | Jha Rajan Kumar |
| India | Kollam | Suravajhala Prashanth |
| India | Haryana | Chakrabarti Sasanka |
| India | New Delhi | Thakkar Himani |
| India | Varanasi | Sonkar Vijay K |
| India | New Delhi | Vincent Vinnyfred |
| Iran | Tehran | Shirazi Reza |
| Iran | Tehran | Safaei Zahra |
| Ireland | Dublin | Porter Richard K |
| Ireland | Dublin | O'Gorman Donal |
| Israel | Beer Sheva | Mishmar Dan |
| Israel | Jerusalem | Saada Reisch Ann |
| Israel | Rishon Le Zion | Hachmo Yafit |
| Israel | Zrifin | Sova Marina |
| Italia | Udine | Salvadego Desy |
| Italien | Padova PD | Szabo Ildiko |
| Italy | Padova | Viscomi Carlo |
| Italy | Ancona | Battino Maurizio |
| Italy | Padova | Bernardi Paolo |
| Italy | Bologna | Genova Maria Luisa |
| Italy | Bolzano | Zanon Alessandra |
| Italy | Campobasso | Pallotta Maria Luigia |
| Italy | Rome | Scatena Roberto |
| Italy | Bologna | Paterlini S |
| Italy | Milano | Bottoni P |
| Italy | St. Lorenzen | Harrison David K |
| Italy | Bologna | Porcelli AM |
| Italy | Catania | Magri Andrea |
| Italy | Catania | Messina Angela |
| Italy | Udine | Grassi B |
| Italy | Monserrato | Isola Raffaella |
| Italy | Catania | De Pinto Vito |
| Italy | Padova | Fernandez-Vizarra Erika |
| Italy | Bologna | Gasparre G |
| Italy | Milan | Clementi Emilio |
| Italy | Bolzano/Bozen | Volani Chiara |
| Italy | Bari | Attimonelli M |
| Japan | Itabashi-ku Tokyo | Tanaka Masashi |
| Japan | Sapporo | Matsumoto J |
| Kitzbühel | Innsbruck | Fischer Michael J |
| Korea | Seoul | Pak Youngmi Kim |
| Korea | Incheon | Kwak Hyo Bum |
| Korea (South) | Seoul | Lee Hong Kyu |
| Latvia | Riga | Svalbe Baiba |
| Latvia | Riga | Zvejniece Liga |
| Latvia | Riga | Stelfa Gundega |
| Latvia | Riga | Liepins Edgars |
| Latvia | Riga | Jansone Baiba |
| Latvia | Riga | Volska Kristine |
| Latvia | Riga | Inashkina I |
| Latvia | Riga | Dambrova Maija |
| Latvia | Riga | Vilks Karlis |
| Luxembourg | Strassen | Devaux Yvan |
| Malaysia | Kuala Lumpur | Hassan Hazirah |
| Malta | Msida | Vella Joanna |
| Mexico | Tlalpan | Rodriguez-Enriquez Sara |
| Mexico | Mexico City | Aparicio Trejo OE |
| México | San Pedro Garza García, Nuevo Leon | Garcia-Rivas Gerardo |
| Netherlands | Maastricht | Grevendonk L |
| Netherlands | GA Wageningen | Grefte Sander |
| New Zealand | Auckland | Kaur Sarbjot |
| New Zealand | Auckland | Ward Marie Louise |
| New Zealand | Auckland | Hickey Anthony J |
| New Zealand | Wellington | Berridge Michael V |
| Norway | Bergen | Dyrstad Sissel E |
| Norway | Oslo | Eide L |
| Norway | Bergen | Haavik Jan |
| Norway | Trondheim | Smenes Benedikte Therese |
| Norway | Trondheim | Rognmo O |
| Norway | Bergen | Hoel Fredrik |
| Norway | Bergen | Roesland Gro Vatne |
| Norway | Tromsoe | Boardman Neoma T |
| Norway | Bergen | Pettersen Nitschke Ina Katrine |
| Norway | Bergen | Blindheim Dan Filip |
| Norway | Bergen | Tronstad Karl Johan |
| Norway | Trondheim | Grill V |
| Norway | Trondheim | Lerfall Joergen |
| Norway | Bergen | Berge Rolf K |
| Poland | Warsaw | Zablocki Krzysztof |
| Poland | Krakow | Dembinska-Kiec Aldona |
| Poland | Lodz | Watala Cezary |
| Poland | Warsaw | Dymkowska Dorota |
| Poland | Warszawa | Jaskiewicz Anna |
| Poland | Lodz | Labieniec-Watala Magdalena |
| Poland | Warsaw | Wasilewski M |
| Poland | Warsaw | Szewczyk Adam |
| Poland | Warsaw | Wieckowski Mariusz R |
| Poland | Warsaw | Bednarczyk Piotr |
| Poland | Warsaw | Kampa Rafal Pawel |
| Poland | Gdańsk | Kaczor Jan Jacek |
| Poland | Warsaw | Sek Aleksandra |
| Poland | Warsaw | Karkucinska-Wieckowska Agnieszka |
| Poland | Poznan | Jarmuszkiewicz Wieslawa |
| Portugal | Coimbra | Santos Diana |
| Portugal | Coimbra | Palmeira Carlos |
| Portugal | Coimbra | Grilo Luis |
| Portugal | Coimbra | Minuzzi Luciele M |
| Portugal | Lisboa | Macedo Maria Paula |
| Portugal | Aveiro | Vitorino Rui Miguel Pinheiro |
| Portugal | Porto | Oliveira Jorge |
| Portugal | Aveiro | Ferreira Rita Maria P |
| Portugal | Porto | Oliveira Pedro Fontes |
| Portugal | Coimbra | Oliveira Paulo J |
| Portugal | Coimbra | Rolo Anabela Pinto |
| Portugal | Cantanhede | Rodrigues Ana Sofia |
| Portugal | Porto | Moreira Bruno P |
| Portugal | Cantanhede | Amorim Ricardo |
| Portugal | Porto | Beleza Jorge |
| Portugal | Coimbra | Dias Candida |
| Portugal | Porto | Crisostomo Luis |
| Portugal | Cantanhede | Teodoro Joao Soeiro |
| Portugal | Porto | Alves Marco G |
| Portugal | Porto | Silva Ana Maria |
| Portugal | Coimbra | Duarte Filipe Valente |
| Qatar | Doha | Guarch Meritxell Espino |
| Republic of Ghana | Cape Coast | Tei BN |
| Republic of Korea | Busan | Kim Hyoung Kyu |
| Republic of Korea | Busan | Han Jin |
| Republic of Korea | Daejeon | Park SH |
| Republic of Korea | Yuseong-gu Daejeon | Quang Don T |
| Republic of Serbia | Belgrade | Lalic Nebojsa M |
| Republic of Serbia | Belgrade | Krako Jakovljevic Nina |
| Republic of Serbia | Novi Sad | Andric Silvana |
| Russia | Moscow | Pavlova Nadia |
| Russia | Moscow | Vinogradov Andrey D |
| Russia | Yoshkar-Ola | Dubinin M |
| Serbia | Belgrade | Misirkic Marjanovic Maja |
| Serbia | Belgrade | Djordjevic M |
| Serbia | Belgrade | Savkovic U |
| Serbia | Belgrade | Mandic M |
| Singapore | Singapore | Singh Brijesh Kumar |
| Singapore | Singapore | Hausenloy Derek J |
| Slovakia | Bratislava | Sumbalova Zuzana |
| Slovakia | Košice | Cizmarova Beata |
| Slovakia | Bratislava 45 | Ferko Miroslav |
| Spain | Barcelona | Garcia-Roves Pablo Miguel |
| Spain | Barcelona | Gil J |
| Spain | Madrid | Hernansanz-Agustin Pablo |
| Spain | A Coruña | Blanco FJ |
| Spain | Granada | Hidalgo-Gutierrez A |
| Spain | Madrid | Moran M |
| Spain | Barcelona | Gama Perez Pau |
| Spain | Granada | Quiles JL |
| Spain | Salamanca | Almeida Angeles |
| Spain | Las Palmas de Gran Canaria | Calbet Jose AL |
| Spain | Madrid | Rial Eduardo |
| Spain | Valencia | Casado Pinna Marta |
| Spain | Madrid | Aragones Lopez J |
| Spain | Sevilla | Bustos Matilde |
| Spain | Barcelona | De la Torre Lara J |
| Spain | A Coruña | Mayan MD |
| Spain | Barcelona | Bravo-Sagua Roberto |
| Spain | Barcelona | Goncalo Teixeira da Silva Rui |
| Spain | Armilla (Granada) | Fernandez-Ortiz Marisol |
| Spain | Barcelona | Zorzano Antonio |
| Spain | Barcelona | Perales Jose Carles |
| Sweden | Lund | Ehinger Johannes K |
| Sweden | Stockholm | Nedergaard J |
| Sweden | Stockholm | Cardinale Daniele A |
| Sweden | Stockholm | Shabalina Irina G |
| Sweden | Stockholm | Larsen Filip J |
| Sweden | Gothenburg | Bhuvanachandran NS |
| Switzerland | Lausanne | Burtscher Johannes |
| Switzerland | Lausanne | Sandi Carmen |
| Switzerland | Zürich | Zaugg Kathrin |
| Switzerland | Bern | Wyss RK |
| Switzerland | Basel | Fornaro Mara |
| Switzerland | Lausanne | Donnelly Chris |
| Switzerland | Basel | Bouitbir Jamal |
| Switzerland | Lausanne | Morato Fornaguera L |
| Switzerland | Lausanne | Place Nicolas |
| Switzerland | Bern | Nuoffer Jean-Marc |
| Switzerland | Lausanne | Canto Alvarez Carles |
| Switzerland | Zurich | Gorr Thomas A |
| Switzerland | Lausanne | Weger M |
| Switzerland | Lausanne | Zanou Nadege |
| Switzerland | Lausanne | Amati Francesca |
| Switzerland | Lausanne | Zalachoras I |
| Switzerland | Lausanne | Lagarrigue Sylviane |
| Switzerland | Lausanne | Gebara E |
| Taiwan | Changhua City | Wei Yau-Huei |
| The Netherlands | Groningen | Bakker Barbara M |
| The Netherlands | Maastricht | Schrauwen Patrick |
| The Netherlands | Maastricht | Nabben Miranda |
| The Netherlands | BT Amsterdam | Wuest Rob CI |
| The Netherlands | Delft | McMillan Duncan GG |
| UK | London | Lane Nick |
| UK | London | Rodriguez Enrique |
| UK | Cambridge | Brown Guy C |
| UK | London | Bettinazzi Stefano |
| UK | Canterbury, Kent | Gourlay Campbell W |
| USA | Kansas City | Thyfault John P |
| USA | Fairbanks | Coker Robert H |
| USA | Fort Collins | Li Puma Lance C |
| USA | Fayetteville | Rao RR |
| USA | Akron | Cohen Bruce H |
| USA | Baton Rouge | Hand Steven C |
| USA | East Lansing | Zhang Yizhu |
| USA | Wakefield | Varricchio Frederick |
| USA | Davis | Hellgren Kim T |
| USA | Orlando | Pino Maria F |
| USA | East Lansing | Bazil Jason N |
| USA | Los Angeles | Kwast Kurt E |
| USA | Nashville | Wasserman David H |
| USA | Little Rock | Borsheim Elisabet |
| USA | Little Rock | MacMillan-Crow Lee Ann |
| USA | Philadelphia | Falk Marni J |
| USA | Winston-Salem | Tomar Dhanendra |
| USA | La Jolla | Schilling Jan M |
| USA | Ann Arbor | Tyrrell Daniel J |
| USA | Chicago | Dayanidhi Sudarshan |
| USA | Aurora | Moreau Kerrie |
| USA | Galveston | Durham William J |
| USA | Greenville | Neufer P Darrell |
| USA | Corvallis | Batterson Philip M |
| USA | San Diego | Patel Hemal H |
| USA | Omaha | Kim Julian KS |
| USA | Louisville | Menze Michael A |
| USA | Warminster | Orynbayeva Zulfiya |
| USA | Little Rock | Parajuli Nirmala |
| USA | Portland | Garlid Keith D |
| USA | Philadelphia | Ganetzky Rebecca |
| USA | Pittsburgh | Jurczak Michael J |
| USA | Indianapolis | Brozinick Joseph T |
| USA | New York | Galkin Alexander |
| USA | Cambridge | Wohlwend Martin |
| USA | Needham | Brown David A |
| USA | Atlanta | Jang Young Charles |
| USA | Provo | Hancock Chad R |
| USA | Miami | Fontanesi F |
| USA | Boston | Kristal Bruce S |
| USA | Iowa City | Wagner Brett A |
| USA | Aurora | Ford Ellen |
| USA | Winston-Salem | Ahn Bumsoo |
| USA | Pittsburgh | Prochownik Edward V |
| USA | St. Louis | Abumrad Nada A |
| USA | Jupiter | Trivigno Catherine |
| USA | Cleveland | Hoppel Charles L |
| USA | Stillwater | Davis Michael S |
| USA | Birmingham | Moellering Douglas R |
| USA | Fort Collins | Chicco Adam J |
| USA | Milwaukee | Dash Ranjan K |
| USA | Torrance | Rossiter Harry B |
| USA | San Diego; La Jolla | Molina Anthony JA |
| USA | Corvallis | Newsom Sean A |
| USA | Bethesda | Sharma Pushpa |
| USA | Orlando | Sparks Lauren M |
| USA | Columbus | Sims Carrie A |
| USA | Rochester | Nair K Sreekumaran |
| USA | Philadelphia | Jang David H |
| USA | Davis | Adams Sean H |
| USA | New Haven | Cavalcanti-de-Albuquerque Joao Paulo |
| USA | St. Louis | Pietka Terri A |
| USA | Gainesville | Leeuwenburgh Christiaan |
| USA | Norfolk | Lai Nicola |
| USA | Seattle | Marcinek DJ |
| USA | San Diego | Cannon Daniel T |
| USA | Puskarich Michael A | |
| USA | Stanford | Stary Creed |
| USA | Durham | Li Pingan Andy |
| USA | Philadelphia | Towheed Atif |
| USA | Cleveland | Tandler Bernard |
| USA | Berkeley | Williams Caroline M |
| USA | Winston-Salem | Gonzalez-Armenta Jenny L |
| USA | Corvallis | Robinson Matthew M |
| USA | Rochester | Lanza Ian R |
| USA | Orlando | Coen Paul M |
| USA | Colorado Springs | Jacobs RA |
| USA | Iowa City | Chaurasia Bhagirath |
| USA | Gainesville | Neyroud D |
| USA | Denton | Dzialowski Edward M |
| USA | Kansas City | Swerdlow Russell H |
| USA | Fairbanks | O'Brien Kristin |
| USA | La Jolla | Valentine Joseph Marco |
| USA | Denver | Van Hove Johan |
| ... further results | ||
WG1 Management
Tasks
- Opening of the database to the research public for data search and moderated entry of new data sets.
- Development of interlaboratory proficiency ring test, of recommendation/certification reports and of a MITOEAGLE proficiency training module (with WG5).
- Sample distribution, ring test experiments (MITOEAGLE-proficiency training on two different points of time, 6 months apart), data analysis by experimenters and MITOEAGLE-proficiency test manager.
- Reports and publication: Reports with specific results in comparison with an anonymous data summary, and joint publication. Individual reports may be complemented by recommendations, and are summarized in an informal certification report (move to WG5).
- Instrumental platform comparison with reference sample applying comparable measurements.
- Draft, dissemination, collection and final formulation of a manuscript on concepts and terminology of mitochondrial physiology (with WG5).
- Collect and discuss procedures and experimental protocols for the evaluation of mitochondrial capacities and create a library of protocols: A database comprising reference SUIT protocols, standard experimental media, and detailed instructions for OXPHOS analysis is assembled.
- MITOEAGLE data management system (DMS) development: The DMS for further data preprocessing. The DMS will enable management and analysis of instrument-specific and project-specific data.
- MITOEAGLE database (DB) development: DB will facilitate high-level project specific analysis as well as meta-analyses across projects, visualization of project specific data as well as integrative analyses of datasets for various proprietary and/or publicly available datasets.
- Testing of developed software components regarding functionality and usability, modification and implementation. Deployment for routine testing in the labs of our strategic partners.
- Data input: Upload of datasets generated through WG 2-4 in internal lab developments, results of the proficiency ring test, and during application module developments. In addition, the database will be populated with available proprietary and public datasets.
- Develop recommendations for quality control, data reporting and data sharing beyond the published record. Develop concepts on Open Access and institutionalized service for data management, data mining and health-care conforming standards of data interpretation (move to WG5).
- Provide a summary on strategic dissemination and an education programme for MITOEAGLE (move to WG5).
Milestones
- Data sets entered in standardized format and database opened for the public
- Protocols for ring-tests and standard analysis and report scheme defined
- MITOEAGLE proficiency training workshop finished (move to WG5)
- Analysis is completed and joint publication submitted (with WG5)
- Publication of paper on concepts and terminology (with WG5)
- Library of protocols online
- Publication of recommended protocols and procedures
- Guidelines for acquisition, evaluation and data documentation available (with WG5)
- MITOEAGLE data management system finished
- MITOEAGLE database ready for use
- Software modifications incorporated following extensive tests
- Publication of MITOEAGLE-KMP
- Recommendations on data acquisition, evaluation and management published (with WG5)
- Concepts on Open Access and institutionalized service for data management, data mining and health-care conforming standards of data interpretation published (move to WG5)
Deliverables
- Guidelines for future research and recommendations for the evaluation of respiratory characteristics in human respirometric samples (with WG5)
- A database on mt fitness evaluated with human cells and tissues
- Training of researchers towards improved reproducibility of sample preparation and respirometric evaluation (move to WG5)
- Qualitative and quantitative evaluation of results obtained in ongoing studies by comparison with a reference sample
- An expanded MITOEAGLE database
- A joint publication documenting the results of the MITOEAGLE-PT (with WG5)
- A multi-authored publication towards a unification of concepts and nomenclature in mitochondrial physiology (with WG5)
- A library of protocols for sample preparation and examination by respirometry
- A multi-authored publication with recommendations of comparable standard protocols and procedures (with WG5)
- Guidelines as to the optimum use of a reference sample for determination of respiration and related parameters of interest
- The MITOEAGLE data management system
- A continuously updated MITOEAGLE database for the use as reference data and guideline towards application of optimized protocols, ultimately leading towards standardization.
- A joint publication on the use of the MITOEAGLE database and management system (with WG5)
- An education programme for the use of MITOEAGLE database and management system (move to WG5).
WG1 Feedback
- We are mostly interested in two WG: (1) Since we are working mostly with heart and muscles, we are interested in WG 2 - MITOEAGLE data repository in muscle and other tissues. (2) WG 1 - Standard operating procedures and user requirement document: Protocols, terminology, documentation. Since we have an expertise in fatty acid metabolism, we are specially interested in experimental protocols for the evaluation of mitochondrial capacities regarding fatty acid metabolism. Of course our parts of the WG are also in our interest. - Marina Makrecka-Kuka, Edgars Liepinsh (2016).
- My role in Mito-EAGLE would be mainly in WG 2 and 4, with a focus on skeletal and cardiac muscle and cultured cardiomyocytes and endothelial cells. I would particularly like to bridge the gap between the group leaders and young investigators, trying to represent the young people who are the active users of the OROBOROS equipment. This will fit in nicely with the goals in WG1. ... Major congratulations on obtaining this beautiful collaborative grant! Very glad to see this network getting the recognition it deserves! Looking forward to being part of this! - Rob C Wuest (2016).
- Please, find enclosed my declaration to participate with my team in the following workgroups: WG1, WG2, WG3, WG4. We are now involved and have been in not a distant past – in studying of the following cell /tissue types:
- Blood cells, with particular emphasis on platelets and lymphocytes – isolated from animals and humans.
- Endothelial cells, fibroblasts, neurocytes (neuroblastoma), astrocytes, cancer cell lines – predominantly cultured.
- Hepatocytes, cardiomyocytes, cell isolated from brain – isolated from lab animals. - Cezary Watala (2016).
- I have already contacted the portuguese CNC to nominate me for the MC of theaction. Concerning my active participation, taking into account the type of work we do in my lab I could be included in all WGs. As you know we work with mitochondria from liver, muscle and fat, as well as cultured cells from different origins (WG2-4). The elaboration of SOPs (WG1) we can certainly contribute (see the book I edited on Mitochondrial Bioenergetics and in which you contribute a chapter). I believe that everybody can be part of the WG5, but as the coordinators of the action, you and Sandra should decide what is best for the success of the action. - Carlos Palmeira (2016).
- I would propose my participation in the WG1: Standard operating procedures and user requirement document: Protocols, terminology, documentation, considering that the goal of WG1 is to establish an integrated and analytic environment linking mitochondrial respiration with external data (cell physiology, anthropometry, spiroergometry, lifestyle, genomics) in the context of physical fitness evaluation and clinical diagnostics. I could give my active contribution in the phase of making platform that will aggregate the Action’s complete knowledge and provides the ability to make diagnostic profiling associated with diseases in particular tasks:
- Develop reccomendations for quality control, data reporting and data sharing beyond the published record. Develop concepts on Open Access and institutionalised service for data management, data mining and health-care conforming standards of data interpretation
- Provide a summary on strategic dissemination and an education programme for MITOEAGLE.
- Considering that we are in process of buying the oxygraph, thus broading capacities of my lab, I would be happy to open the door of my new lab for the innovative inter-laboratory ring test experiments and together with my team contribute in that phase of the MITOEAGLE Action. I look forward to the establishment of MitoGlobal EAGLE network where, as stated in the MoU, researchers will collaborate on mapping mitochondrial physiology and medicine. The creation of such a unique well-coordinated network of senior researchers and young investigators will definitely put an added value to existing data and publications, will increase quality and validity of the research, will bring new possibilities of data sharing and data mining and will hopefully bring us to the era of more economical research by saving resources through standardised and harmnonised protocols, where the measuremnts will not be wasted due to lack of comparability. - Nebojsa Lalic (2016).
- Our research interest is oriented to reproductive endocrinology; especially to mechanisms that govern steroidogenesis of testicular Leydig cells in disturbed homeostasis. We use several in vivo models:
- stress -/+ systemic or intratesticular blockade of different stress receptors (alpha1ADR; betaADR; GR)
- hypogonadal-hypogonadism -/+ testosterone-replacement therapy
- androgens anabolics treatment -/+ blockade od AR
- aged rats -/+ Viagra-treatment
- biological clock - Silvana Andric (2016).
- I took a look to the MoU and the different working groups. You know well my research focus and having this into account I am interested in actions related to Working group 1 (Respirometric reference protocols, Interlaboratory proficiency test, Instrumental platforms). Working group 2 and 3: I have generated multiple mitochondrial respiration data sets in different mouse tissues (mainly in skeletal muscle, hypothalamus, liver and white adipose tissue). Currently we are also performing these assays in white adipose tissue from human patients. Hence I am interested in all milestones of WG 2 and 3. WG 5 is related to WG 1 and as you know I have interest in training, dissemination and also together with my collaborators (if needed) we could help with data sets and a data interpretation. Overall, I have a general interest on this Action and my active role in MITOEAGLE could be discussed. I could be of help participating in some of the tasks where my expertise and interest fit best. We will be forming and interdisciplinary research group with four group leaders: JC Perales, R Guimera, M Sales-Pardo and PM Garcia-Roves with two different affiliations, University of Barcelona (JCP and PMG-R - Energy metabolism) and University Rovira I Virgili (RG and MS – Data base, statistics, data mining). - Pablo M Garcia-Roves (2016).
- I will be pleased to contribute to the tasks of WG1 by developing interlaboratory proficiency tests and instrumental platform comparison. - Maria Luisa Genova (2016).
Projects in progress
- Interlaboratory proficiency ring test
- Testing of software components
- Concepts and terminology on mitochondrial physiology
- Procedures and experimental protocols for the evaluation of mitochondrial capacities, library of protocols
- Data management system (DMS)
- Database
Next steps
- Best practices for implementation of analytical and process standards.
References
- General requirements for the competence of testing and calibration laboratories » ISO/IEC 17025:2005