Difference between revisions of "Pyruvate"
| (7 intermediate revisions by the same user not shown) | |||
| Line 8: | Line 8: | ||
== Application in [[HRR]] == | == Application in [[HRR]] == | ||
{{Chemical_description | |||
|abbr=P | |||
|trivial name=Pyruvate | |||
|complete name=pyruvic acid, sodium salt | |||
|chem formula=C<sub>3</sub>H<sub>3</sub>O<sub>3</sub>Na | |||
|molar mass=110.0 | |||
|vendor=Sigma-Aldrich | |||
|product number=P2256 | |||
|store at=4 °C | |||
|sensitivity= | |||
|cas=113-24-6 | |||
|h statements=H317, H319 | |||
|h info=may cause an allergic skin reaction, causes serious eye irritation | |||
}}<!--::: '''P: Pyruvate''' (pyruvic acid, sodium salt, C<sub>3</sub>H<sub>3</sub>O<sub>3</sub>Na); Sigma P 2256, 25 g, store at 4 °C; M = 110.0 g·mol<sup>-1</sup>--> | |||
:::: It is possible to weigh the powder beforehand in the Eppendorf-type tubes and store these tubes at 4 °C, to be diluted only on the day of use. | |||
:::: After addition of H<sub>2</sub>O the pH of the Pyruvate solution is about 6. This is acceptable without pH-adjustment, because the titrated volumes are small and reaction media are buffered. | |||
::: | :::: 2021-03: The preparation instructions were updated to take the volume of the solute (P) into account (see: [[Volume_of_the_solute| Volume of the solute]]). The concentrations prepared following the former instructions (see Discussion section) are sufficiently high for SUIT protocol titrations. | ||
: | |||
:::: '''Preparation of 2 M stock solution''' (dissolved in H<sub>2</sub>O) for use in '''2-mL O2k-chamber''': | :::: '''Preparation of 2 M stock solution''' (200 µL, dissolved in H<sub>2</sub>O) for use in '''2-mL O2k-chamber''': | ||
::::# Prepare fresh everyday. | ::::# Prepare fresh everyday. | ||
::::# Weigh 44 mg of pyruvic acid directly into a 0.5 mL Eppendorf tube. | ::::# Weigh 44 mg of pyruvic acid directly into a 0.5 mL Eppendorf tube. | ||
::::# Add | ::::# Add 180 µL H<sub>2</sub>O. | ||
:::» '''O2k manual titrations''' [[MiPNet09.12 O2k-Titrations]] | :::» '''O2k manual titrations''' [[MiPNet09.12 O2k-Titrations]] | ||
| Line 26: | Line 40: | ||
:::: '''Preparation of 2.5 M stock solution''' (dissolved in H<sub>2</sub>O) for use in '''0.5-mL O2k-chamber''': | :::: '''Preparation of 2.5 M stock solution''' (200 µL, dissolved in H<sub>2</sub>O) for use in '''0.5-mL O2k-chamber''': | ||
::::# Prepare fresh everyday. | ::::# Prepare fresh everyday. | ||
::::# Weigh 55 mg of pyruvic acid directly into a 0.5 mL Eppendorf tube. | ::::# Weigh 55 mg of pyruvic acid directly into a 0.5 mL Eppendorf tube. | ||
::::# Add | ::::# Add 175 µL H<sub>2</sub>O. | ||
:::» '''O2k manual titrations''' [[MiPNet09.12 O2k-Titrations]] | :::» '''O2k manual titrations''' [[MiPNet09.12 O2k-Titrations]] | ||
::::* Titration volume ('''0.5-mL O2k-chamber'''): 1 µL using a 10 µL Hamilton syringe. | ::::* Titration volume ('''0.5-mL O2k-chamber'''): 1 µL using a 10 µL Hamilton syringe. | ||
::::* Final concentration: 5 mM. | ::::* Final concentration: 5 mM. | ||
== Troubleshooting == | == Troubleshooting == | ||
Latest revision as of 11:20, 17 June 2021
Description
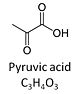
Pyruvic acid, C3H4O3, is an alpha-keto monocarboxylic acid which occurs under physiological conditions mainly as the anion pyruvate-, P, with pKa = 2.5. Pyruvate is formed in glycolysis from phosphoenolpyruvate. In the cytosol, pyruvate is a substrate of lactate dehydrogenase. Pyruvate enters the mitochondrial matrix via a specific low Km' H+/monocarboxylate cotransporter known as the pyruvate carrier. Similarly, the plasma membrane of many cell types has H+/monocarboxylate cotransporter activity and pyruvate can thus be added as a substrate to living cells. In the mt-matrix the oxidative decarboxylation of pyruvate is catalyzed by pyruvate dehydrogenase and yields acetyl-CoA. Pyruvate competitively reverses the inhibition of cytochrome c oxidase by cyanide. Pyruvate is an antioxidant reacting with hydrogen peroxide.
Abbreviation: P
Reference: Gnaiger 2020 BEC MitoPathways, MiPNet09.12 O2k-Titrations
Application in HRR
- P: Pyruvate (pyruvic acid, sodium salt; C3H3O3Na), Sigma-Aldrich: P2256, store at 4 °C, CAS: 113-24-6, M = 110.0 g·mol-1
- Hazard statements: H317, H319; may cause an allergic skin reaction, causes serious eye irritation
- P: Pyruvate (pyruvic acid, sodium salt; C3H3O3Na), Sigma-Aldrich: P2256, store at 4 °C, CAS: 113-24-6, M = 110.0 g·mol-1
- It is possible to weigh the powder beforehand in the Eppendorf-type tubes and store these tubes at 4 °C, to be diluted only on the day of use.
- After addition of H2O the pH of the Pyruvate solution is about 6. This is acceptable without pH-adjustment, because the titrated volumes are small and reaction media are buffered.
- 2021-03: The preparation instructions were updated to take the volume of the solute (P) into account (see: Volume of the solute). The concentrations prepared following the former instructions (see Discussion section) are sufficiently high for SUIT protocol titrations.
- Preparation of 2 M stock solution (200 µL, dissolved in H2O) for use in 2-mL O2k-chamber:
- Prepare fresh everyday.
- Weigh 44 mg of pyruvic acid directly into a 0.5 mL Eppendorf tube.
- Add 180 µL H2O.
- » O2k manual titrations MiPNet09.12 O2k-Titrations
- Titration volume (2-mL O2k-chamber): 5 µL using a 25 µL Hamilton syringe.
- Final concentration: 5 mM.
- Preparation of 2.5 M stock solution (200 µL, dissolved in H2O) for use in 0.5-mL O2k-chamber:
- Prepare fresh everyday.
- Weigh 55 mg of pyruvic acid directly into a 0.5 mL Eppendorf tube.
- Add 175 µL H2O.
- » O2k manual titrations MiPNet09.12 O2k-Titrations
- Titration volume (0.5-mL O2k-chamber): 1 µL using a 10 µL Hamilton syringe.
- Final concentration: 5 mM.
Troubleshooting
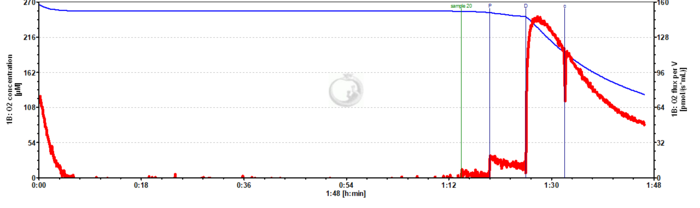
Unstable respiration while using pyruvate as the only substrate
- Customer ID: AU Melbourne White C
- Question:
- I am evaluating mitochondrial respiration from Drosophila melanogaster using Pyruvate, ADP, and Cytochrome C. However, I do not achieve a steady state level in OXPHOS.
- Any advice would be appreciated. The data is attached (2019-07-17).
- Answer:Pyruvate alone is not sufficient to support NADH-linked respiration. In order to do so you need to combine pyruvate with at least a second NADH-linked substrate (e.g. Malate) or use a more complex combination of substrates (e.g., Pyruvate&Glutamate&Malate). See Fig. 5.9. in Gnaiger 2020 BEC MitoPathways
- Additionally, you may consult some of the publications from Drosophila melanogaster mitochondria: O2k-Publications:_Drosophila
MitoPedia topics:
Substrate and metabolite