Difference between revisions of "Superoxide"
From Bioblast
| Line 1: | Line 1: | ||
{{MitoPedia | {{MitoPedia | ||
|abbr=O<sub>2</sub><sup>- | |abbr=O<sub>2</sub><sup>•-</sup> | ||
|description=[[File:O2-.jpg|left|60px|Superoxide anion]] | |description=[[File:O2-.jpg|left|60px|Superoxide anion]] | ||
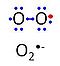
'''Superoxide anion''', O<sub>2</sub><sup>•-</sup>, is a free radical formed in a one-electron reduction of molecular [[oxygen]] (red bullet in the figure), yielding a negatively charged molecule with a single unpaired electron (blue bullet on the left). It is highly reactive with organic compounds, and its intracellular concentration is kept under control by [[superoxide dismutase]]. | '''Superoxide anion''', O<sub>2</sub><sup>•-</sup>, is a free radical formed in a one-electron reduction of molecular [[oxygen]] (red bullet in the figure), yielding a negatively charged molecule with a single unpaired electron (blue bullet on the left). It is highly reactive with organic compounds, and its intracellular concentration is kept under control by [[superoxide dismutase]]. | ||
Latest revision as of 03:57, 16 April 2014
Description
Superoxide anion, O2•-, is a free radical formed in a one-electron reduction of molecular oxygen (red bullet in the figure), yielding a negatively charged molecule with a single unpaired electron (blue bullet on the left). It is highly reactive with organic compounds, and its intracellular concentration is kept under control by superoxide dismutase.
Abbreviation: O2•-
Reference: Fridovich_1997_J Biol Chem
MitoPedia topics: Substrate and metabolite