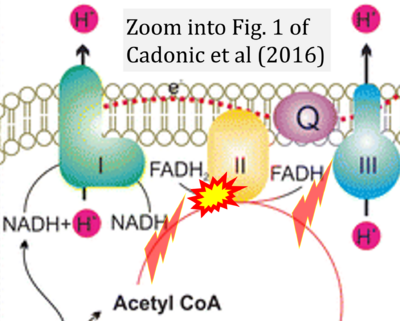
Cadonic 2016 Mol Neurobiol
| Cadonic C, Sabbir MG, Albensi BC (2016) Mechanisms of mitochondrial dysfunction in Alzheimer's disease. Mol Neurobiol 53:6078-90. https://doi.org/10.1007/s12035-015-9515-5 |
Cadonic C, Sabbir MG, Albensi BC (2016) Mol Neurobiol
Abstract: Mitochondria are the primary source for energy generation in the cell, which manifests itself in the form of the adenosine triphosphate (ATP). Nicotinamide dinucleotide (NADH) molecules are the first to enter the so-called electron transport chain or ETC of the mitochondria. The ETC represents a chain of reducing agents organized into four major protein-metal complexes (I-IV) that utilize the flow of electrons to drive the production of ATP. An additional integral protein that is related to oxidative phosphorylation is ATP synthase, referred to as complex V. Complex V carries out ATP synthesis as a result of the electron flow through the ETC. The coupling of electron flow from NADH to molecular oxygen to the production of ATP represents a process known as oxidative phosphorylation. In this review, we describe mainly the bioenergetic properties of mitochondria, such as those found in the ETC that may be altered in Alzheimer's disease (AD). Increasing evidence points to several mitochondrial functions that are affected in AD. Furthermore, it is becoming apparent that mitochondria are a potential target for treatment in early-stage AD. With growing interest in the mitochondria as a target for AD, it has been hypothesized that deficit in this organelle may be at the heart of the progression of AD itself. The role of mitochondria in AD may be significant and is emerging as a main area of AD research.
• Bioblast editor: Gnaiger E
Correction: FADH2 and Complex II
- FADH2 is shown as the substrate feeding electrons into Complex II (CII). This is wrong and requires correction - for details see Gnaiger (2024).
- Gnaiger E (2024) Complex II ambiguities ― FADH2 in the electron transfer system. J Biol Chem 300:105470. https://doi.org/10.1016/j.jbc.2023.105470 - »Bioblast link«
Hydrogen ion ambiguities in the electron transfer system
Communicated by Gnaiger E (2023-10-08) last update 2023-11-10
- Electron (e-) transfer linked to hydrogen ion (hydron; H+) transfer is a fundamental concept in the field of bioenergetics, critical for understanding redox-coupled energy transformations.
- However, the current literature contains inconsistencies regarding H+ formation on the negative side of bioenergetic membranes, such as the matrix side of the mitochondrial inner membrane, when NADH is oxidized during oxidative phosphorylation (OXPHOS). Ambiguities arise when examining the oxidation of NADH by respiratory Complex I or succinate by Complex II.
- Oxidation of NADH or succinate involves a two-electron transfer of 2{H++e-} to FMN or FAD, respectively. Figures indicating a single electron e- transferred from NADH or succinate lack accuracy.
- The oxidized NAD+ is distinguished from NAD indicating nicotinamide adenine dinucleotide independent of oxidation state.
- NADH + H+ → NAD+ +2{H++e-} is the oxidation half-reaction in this H+-linked electron transfer represented as 2{H++e-} (Gnaiger 2023). Putative H+ formation shown as NADH → NAD+ + H+ conflicts with chemiosmotic coupling stoichiometries between H+ translocation across the coupling membrane and electron transfer to oxygen. Ensuring clarity in this complex field is imperative to tackle the apparent ambiguity crisis and prevent confusion, particularly in light of the increasing number of interdisciplinary publications on bioenergetics concerning diagnostic and clinical applications of OXPHOS analysis.
Labels:
Enzyme: Complex II;succinate dehydrogenase