Burgin 2020 FEBS Lett
| Burgin HJ, McKenzie M (2020) Understanding the role of OXPHOS dysfunction in the pathogenesis of ECHS1 deficiency. FEBS Lett 594:590-610. https://doi.org/10.1002/1873-3468.13735 |
Burgin HJ, McKenzie M (2020) FEBS Lett
Abstract: Mitochondria provide the main source of energy for eukaryotic cells, oxidizing fatty acids and sugars to generate ATP. Mitochondrial fatty acid β-oxidation (FAO) and oxidative phosphorylation (OXPHOS) are two key pathways involved in this process. Disruption of FAO can cause human disease, with patients commonly presenting with liver failure, hypoketotic glycaemia and rhabdomyolysis. However, patients with deficiencies in the FAO enzyme short-chain enoyl-CoA hydratase 1 (ECHS1) are typically diagnosed with Leigh syndrome, a lethal form of subacute necrotizing encephalomyelopathy that is normally associated with OXPHOS dysfunction. Furthermore, some ECHS1-deficient patients also exhibit secondary OXPHOS defects. This sequela of FAO disorders has long been thought to be caused by the accumulation of inhibitory fatty acid intermediates. However, new evidence suggests that the mechanisms involved are more complex, and that disruption of OXPHOS protein complex biogenesis and/or stability is also involved. In this review, we examine the clinical, biochemical and genetic features of all ECHS1-deficient patients described to date. In particular, we consider the secondary OXPHOS defects associated with ECHS1 deficiency and discuss their possible contribution to disease pathogenesis.
• Bioblast editor: Gnaiger E
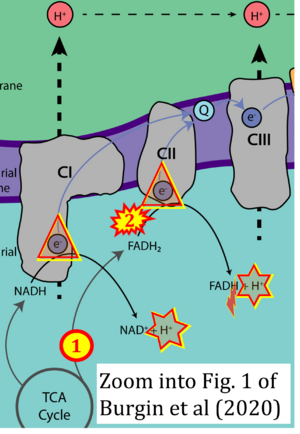
Correction: FADH2 and Complex II
- FADH2 is shown as the substrate feeding electrons into Complex II (CII). This is wrong and requires correction - for details see Gnaiger (2024).
- Gnaiger E (2024) Complex II ambiguities ― FADH2 in the electron transfer system. J Biol Chem 300:105470. https://doi.org/10.1016/j.jbc.2023.105470 - »Bioblast link«
Hydrogen ion ambiguities in the electron transfer system
Communicated by Gnaiger E (2023-10-08) last update 2023-11-10
- Electron (e-) transfer linked to hydrogen ion (hydron; H+) transfer is a fundamental concept in the field of bioenergetics, critical for understanding redox-coupled energy transformations.
- However, the current literature contains inconsistencies regarding H+ formation on the negative side of bioenergetic membranes, such as the matrix side of the mitochondrial inner membrane, when NADH is oxidized during oxidative phosphorylation (OXPHOS). Ambiguities arise when examining the oxidation of NADH by respiratory Complex I or succinate by Complex II.
- Oxidation of NADH or succinate involves a two-electron transfer of 2{H++e-} to FMN or FAD, respectively. Figures indicating a single electron e- transferred from NADH or succinate lack accuracy.
- The oxidized NAD+ is distinguished from NAD indicating nicotinamide adenine dinucleotide independent of oxidation state.
- NADH + H+ → NAD+ +2{H++e-} is the oxidation half-reaction in this H+-linked electron transfer represented as 2{H++e-} (Gnaiger 2023). Putative H+ formation shown as NADH → NAD+ + H+ conflicts with chemiosmotic coupling stoichiometries between H+ translocation across the coupling membrane and electron transfer to oxygen. Ensuring clarity in this complex field is imperative to tackle the apparent ambiguity crisis and prevent confusion, particularly in light of the increasing number of interdisciplinary publications on bioenergetics concerning diagnostic and clinical applications of OXPHOS analysis.