LaMoia 2022 Proc Natl Acad Sci U S A
| LaMoia TE, Butrico GM, Kalpage HA, Goedeke L, Hubbard BT, Vatner DF, Gaspar RC, Zhang XM, Cline GW, Nakahara K, Woo S, Shimada A, Hüttemann M, Shulman GI (2022) Metformin, phenformin, and galegine inhibit complex IV activity and reduce glycerol-derived gluconeogenesis. Proc Natl Acad Sci U S A 119:e2122287119. https://doi.org/10.1073/pnas.2122287119 |
LaMoia TE, Butrico GM, Kalpage HA, Goedeke L, Hubbard BT, Vatner DF, Gaspar RC, Zhang XM, Cline GW, Nakahara K, Woo S, Shimada A, Huettemann M, Shulman GI (2022) Proc Natl Acad Sci U S A
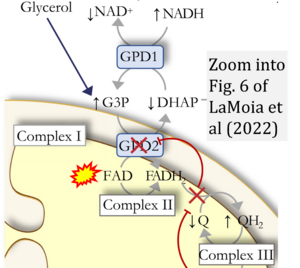
Abstract: Metformin is the most commonly prescribed drug for the treatment of type 2 diabetes mellitus, yet the mechanism by which it lowers plasma glucose concentrations has remained elusive. Most studies to date have attributed metformin's glucose-lowering effects to inhibition of complex I activity. Contrary to this hypothesis, we show that inhibition of complex I activity in vitro and in vivo does not reduce plasma glucose concentrations or inhibit hepatic gluconeogenesis. We go on to show that metformin, and the related guanides/biguanides, phenformin and galegine, inhibit complex IV activity at clinically relevant concentrations, which, in turn, results in inhibition of glycerol-3-phosphate dehydrogenase activity, increased cytosolic redox, and selective inhibition of glycerol-derived hepatic gluconeogenesis both in vitro and in vivo.
• Bioblast editor: Gnaiger E
Labels: MiParea: Pharmacology;toxicology
Enzyme: Complex IV;cytochrome c oxidase
Metformin
Correction: FADH2 and Complex II
- FADH2 is shown as the substrate feeding electrons into Complex II (CII). This is wrong and requires correction - for details see Gnaiger (2024).
- Gnaiger E (2024) Complex II ambiguities ― FADH2 in the electron transfer system. J Biol Chem 300:105470. https://doi.org/10.1016/j.jbc.2023.105470 - »Bioblast link«