Gnaiger 2018 MiPschool Tromso A1
| Mitochondrial states and rates: 1. Electron transfer pathways and respiratory control. 2. Coupling control. |
Link: MitoEAGLE
Gnaiger E (2018)
Event: MiPschool Tromso-Bergen 2018
The MitoEAGLE project aims at establishing a quantitative database on mitochondrial (mt) respiratory physiology. In this context the necessity for harmonizing the terminology has become increasingly apparent. Substrate-uncoupler-inhibitor titrations (SUIT) are applied to experimentally control electron transfer pathways in mitochondrial preparations. Complementary to pathway control states (PCS), coupling control states (CCS: ET, OXPHOS, LEAK) are defined in mt-preparations, and the corresponding respiratory rates are of diagnostic significance [1]. Strategically designed SUIT protocols reveal a diversity of mt-respiratory control patterns and pathway additivity depending on species, organs, cell types, and pathophysiological states, as a hallmark of the transition from bioenergetics to mitochondrial physiology [2]. A rationale for categorizing PCS helps in selecting SUIT protocols according to the specific research question or diagnostic aim, and is essential for interpreting experimental results [3].
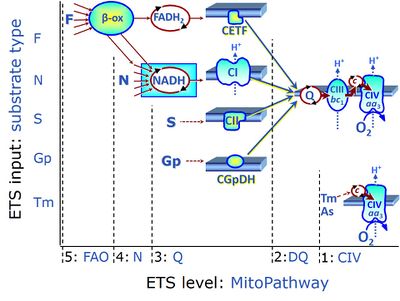
Figure 1 summarizes selected PCS, categorized according to fuel substrate types and the complexity of mitochondrial pathway types at different electron transfer- (ET-) pathway levels. ET-pathway levels are linked to ET-substrate types. The single enzyme step of Complex IV is at level 1. ET-pathway level 2 is stimulated by duroquinol (DQ) feeding electrons into Complex III (CIII) with further electron transfer to CIV and O2. ET-pathway level 3 feeds electrons from succinate to CII, and glycerophosphate (Gp) to GpDH directly upstream of the Q-junction. Electron transfer from type 4 substrates (N) feeds electrons into the N-junction from dehydrogenases and enzyme systems directly upstream of NADH and CI. The requirement of a combined operation of the F-junction and N-junction puts type F substrates to level 5 of pathway integration. F-junction substrates are fatty acids involved in β-oxidation, generating (enzyme-bound) FADH2, the substrate of electron transferring flavoprotein (CETF). In contrast, FADH2 is the product of CII. A N-linked co-substrate (typically malate [5]) is required, and FAO can be inhibited completely by inhibition of Complex I (CI). Under physiological conditions, combinations of the fuel substrate types extend the complexity of PCS, exerting additive or competitive effects on respiratory capacity [2-4]. Analysis of combined NS- versus single N- and S-pathway capacities yields information on pathway interactions and channeling through supercomplex assemblies [4], and leads to a re-evaluation of apparent excess capacities of CIV [6].
Biochemical cell ergometry aims at measurement of JO2,max (compare VO2,max in exercise ergometry of humans and animals) of cell respiration linked to phosphorylation of ADP to ATP. The corresponding OXPHOS-capacity is based on saturating concentrations of ADP, [ADP]*, and inorganic phosphate, [Pi]*, available to the mitochondria. This is metabolically opposite to experimental uncoupling of respiration, which yields noncoupled ET-capacity. Contrasting the concept-driven terminology on CCS (LEAK, OXPHOS, ET) from the historical terminology in bioenergetics (States 1 to 5) provides important insights into the aims and rigorous quality control of diagnostic mitochondrial physiology [1].
We cordially invite scientists and students to support our effort to prepare joint publications for implementing a consistent terminology on respiratory states, to ‘facilitate effective transdisciplinary communication, education, and ultimately further discovery’ and advance the quality and impact of mitochondrial physiology [1].
• Bioblast editor: Gnaiger E
Labels:
Coupling state: LEAK, ET
Affiliations and support
- D. Swarovski Research Lab, Dept. Visceral, Transplant Thoracic Surgery, Medical Univ Innsbruck
- Oroboros Instruments, Innsbruck, Austria
- Supported by K-Regio project MitoFit. Contribution to COST Action CA15203 MitoEAGLE.
References
- MitoEAGLE preprint 2018-09-04(41) Mitochondrial respiratory states and rates: Building blocks of mitochondrial physiology Part 1. - www.mitoeagle.org/index.php/MitoEAGLE_preprint_2018-02-08
- Gnaiger E (2009) Capacity of oxidative phosphorylation in human skeletal muscle. New perspectives of mitochondrial physiology. Int J Biochem Cell Biol 41:1837-45. - »Bioblast link«
- Doerrier C, Garcia-Souza LF, Krumschnabel G, Wohlfarter Y, Mészáros AT, Gnaiger E (2018) High-Resolution FluoRespirometry and OXPHOS protocols for human cells, permeabilized fibers from small biopsies of muscle, and isolated mitochondria. Methods Mol Biol 1782:31-70. - »Bioblast link«
- Gnaiger E (2014) Mitochondrial pathways and respiratory control. An introduction to OXPHOS analysis. 4th ed. Mitochondr Physiol Network 19.12. Oroboros MiPNet Publications, Innsbruck:80 pp. - »Bioblast link«
- Doerrier C, Garcia-Souza LF, Sumbalova Z, Krumschnabel G, Gnaiger E (2018) Evaluation of anaplerotic pathways to avoid artefacts in respirometric measurement of fatty acid oxidation. MiP2018. - »Bioblast link«
- Lemieux H, Blier PU, Gnaiger E (2017) Remodeling pathway control of mitochondrial respiratory capacity by temperature in mouse heart: electron flow through the Q-junction in permeabilized fibers. Sci Rep 7:2840. - »Bioblast link«
Figure
- Figure 1. ET-pathway control states are defined in mitochondrial preparations complementary to coupling control states. From http://www.bioblast.at/index.php/Electron_transfer-pathway_state
Labels: MiParea: Respiration, mt-Awareness
Coupling state: OXPHOS Pathway: F, N, S, Gp, DQ, CIV, NS, Other combinations
Event: Oral