Bottje 2019 Poult Sci
| Bottje WG (2019) Oxidative metabolism and efficiency: the delicate balancing act of mitochondria. Poult Sci 98:4223-30. https://doi.org/10.3382/ps/pey405 |
Abstract: Mitochondria are responsible for roughly 90% of the ATP produced in a cell. A consequence of aerobic metabolism is oxidative stress that results from production of mitochondrial reactive oxygen species (ROS) due to inefficiency of electron transport. Several antioxidant-redox coupled reactions in the mitochondria help minimize oxidative damage in the mitochondria. These redox reactions not only protect mitochondria from oxidative damage but also are important in regulating cellular redox status. Oxidative stress from mitochondrial ROS occurs in broilers in pulmonary hypertension syndrome, heat stress, and in the phenotypic expression of feed efficiency. Low levels of mitochondrial ROS are now recognized to play important roles in signal transduction mechanisms. A topology of ROS production has been reported that indicates that ROS derived from Complex I primarily cause oxidative damage, whereas ROS generated from Complex III are primarily involved in cell signaling. Reverse electron transport, once considered an artifact of in vitro conditions, now plays significant roles in physiological conditions including inflammation, ischemia-reperfusion, muscle differentiation, and energy utilization. Understanding the balancing act that mitochondria play in health and disease will continue to be vital biological component of improving efficiency in animal production.
• Bioblast editor: Gnaiger E
Hydrogen ion ambiguities in the electron transfer system
Communicated by Gnaiger E (2023-10-08) last update 2023-11-10
- Electron (e-) transfer linked to hydrogen ion (hydron; H+) transfer is a fundamental concept in the field of bioenergetics, critical for understanding redox-coupled energy transformations.
- However, the current literature contains inconsistencies regarding H+ formation on the negative side of bioenergetic membranes, such as the matrix side of the mitochondrial inner membrane, when NADH is oxidized during oxidative phosphorylation (OXPHOS). Ambiguities arise when examining the oxidation of NADH by respiratory Complex I or succinate by Complex II.
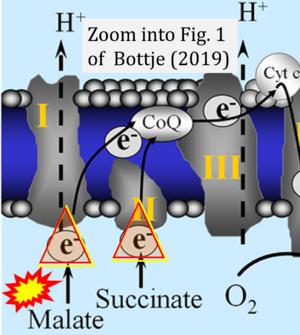
- Oxidation of NADH or succinate involves a two-electron transfer of 2{H++e-} to FMN or FAD, respectively. Figures indicating a single electron e- transferred from NADH or succinate lack accuracy.
- The oxidized NAD+ is distinguished from NAD indicating nicotinamide adenine dinucleotide independent of oxidation state.
- NADH + H+ → NAD+ +2{H++e-} is the oxidation half-reaction in this H+-linked electron transfer represented as 2{H++e-} (Gnaiger 2023). Putative H+ formation shown as NADH → NAD+ + H+ conflicts with chemiosmotic coupling stoichiometries between H+ translocation across the coupling membrane and electron transfer to oxygen. Ensuring clarity in this complex field is imperative to tackle the apparent ambiguity crisis and prevent confusion, particularly in light of the increasing number of interdisciplinary publications on bioenergetics concerning diagnostic and clinical applications of OXPHOS analysis.
- Fig. 1 of Bottje (2019): NADH+H+ should be indicated as substrate of CI compared to succinate as substrate of CII. Their oxidation is a 2-electron reaction.