Nussbaum 2005 J Clin Invest
| Nussbaum RL (2005) Mining yeast in silico unearths a golden nugget for mitochondrial biology. J Clin Invest 115:2689-91. https://doi.org/10.1172/JCI26625 |
Nussbaum RL (2005) J Clin Invest
Abstract: NADH:ubiquinone oxidoreductase (complex I) of the electron transport chain is a multimeric mitochondrial enzyme of approximately 1000 kDa consisting of 46 different proteins encoded by both the mitochondrial and nuclear genomes. Little is known about the cellular mechanisms and protein chaperones that guide its assembly. In this issue of the JCI, Ogilvie et al. use genomic sequence data to compare the proteins produced by yeasts with and without complex I in order to generate a list of proteins whose human orthologs might serve as complex I assembly proteins. The gene encoding one of these candidate proteins, B17.2L, was found to harbor a nonsense mutation in one of 28 patients with a deficiency of complex I. B17.2L associated with subcomplexes that are seen when complex I assembly is incomplete. The research described here combines clever model organism genomics and bioinformatics with sophisticated human molecular and biochemical genetics to identify the first mammalian protein required for the normal assembly of complex I.
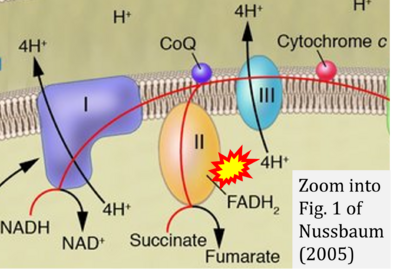
Correction: FADH2 and Complex II
- FADH2 is shown as the substrate feeding electrons into Complex II (CII). This is wrong and requires correction - for details see Gnaiger (2024).
- Gnaiger E (2024) Complex II ambiguities ― FADH2 in the electron transfer system. J Biol Chem 300:105470. https://doi.org/10.1016/j.jbc.2023.105470 - »Bioblast link«
Labels:
Organism: Saccharomyces cerevisiae
Pathway: S