Difference between revisions of "Hatefi 1962 J Biol Chem-XLII"
Harrison DK (talk | contribs) (Created page with "{{Publication |title=Hatefi Y, Haavik AG, Fowler LR, Griffiths DE (1962) Studies on the electron transfer system XLII. Reconstitution of the electron transfer system. J Biol Chem...") |
|||
| (29 intermediate revisions by 7 users not shown) | |||
| Line 1: | Line 1: | ||
{{Publication | {{Publication | ||
|title=Hatefi Y, Haavik AG, Fowler LR, Griffiths DE (1962) Studies on the electron transfer system XLII. Reconstitution of the electron transfer system. J Biol Chem 237: 2661- | |title=Hatefi Y, Haavik AG, Fowler LR, Griffiths DE (1962) Studies on the electron transfer system XLII. Reconstitution of the electron transfer system. J Biol Chem 237:2661-9. https://doi.org/10.1016/S0021-9258(19)73804-6 | ||
|info=[http://www.jbc.org/content/237/8/2661.full.pdf+html PMID:13905326 Open Access] | |info=[http://www.jbc.org/content/237/8/2661.full.pdf+html PMID:13905326 Open Access] | ||
|authors=Hatefi Y, Haavik AG, Fowler LR, Griffiths DE | |authors=Hatefi Y, Haavik AG, Fowler LR, Griffiths DE | ||
|year=1962 | |year=1962 | ||
|journal=J Biol Chem | |journal=J Biol Chem | ||
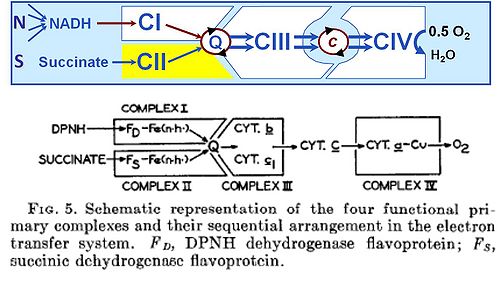
|abstract= | |abstract=1. It has been shown that the electron transfer system in beef heart mitochondria may be reconstituted either totally or in any desired sequential segment by appropriate combinations of two or more of the four primary complexes that have been isolated in highly purified form in this laboratory. | ||
|keywords= | 2. The four enzyme systems that collectively comprise the complete machinery for transfer of electrons from reduced diphosphopyridine nucleotide (DPNH; =NADH) and succinate to oxygen re: I, DPNH-coenzyme Q reductase; II, succinic-coenzyme Q reductase; III, QH2-cytochrome ''c'' reductase; and IV, cytochrome ''c'' reductase. The specific inhibitors of each complex have been studied. | ||
3. By appropriate combinations of the primary complexes the following secondary activities have been reconstituted: V, DPNH-cytochrome ''c'' reductase; VI, succinic-cytochrome ''c'' reductase; VII, DPNH, succinic-cytochrome c reductase; VIII, DPNH oxidase; IX, succinic oxidase; and X, DPNH, succinic oxidase activity. The general oxidation-reduction properties of the reconstituted systems, both in the presence and the absence of the usual specific inhibitors of the electron transfer system, are essentially the same as those found for the same activities in the intact mitochondria and in the integrated particles derived therefrom. | |||
4. The reconstituted activities are quite stable to repeated freezing, thawing, and storage at -2O °C, and for the most part, when once formed, are not dissociated by dilution of the mixture or by centrifugation. The evidence supporting the conclusion that reconstitution necessarily involves a particle-particle interaction is discussed. | |||
|keywords=Electron transfer, DPNH-coenzyme Q reductase, Succinic-coenzyme Q reductase, QH2-cytochrome c reductase, Cytochrome c reductase, Beef heart | |||
}} | }} | ||
[[File:Hatefi 1962 NS 2012.jpg|right|500px|Q-junction]] | |||
== Electron transfer system versus electron transport chain == | |||
:::* ''More details:'' »[[Electron transfer-pathway]] | |||
== Cited by == | |||
{{Template:Cited by Gnaiger 2020 BEC MitoPathways}} | |||
{{Labeling | {{Labeling | ||
|organism= | |organism=Bovines | ||
|tissues= | |tissues=Heart | ||
|preparations=Isolated | |preparations=Isolated mitochondria | ||
|enzymes=Complex II; | |enzymes=Complex II;succinate dehydrogenase, Complex IV;cytochrome c oxidase | ||
| | |topics=Substrate | ||
|couplingstates=ET | |||
|additional=Made history, Electron transfer pathway, BEC 2020.2 | |||
}} | }} | ||
Latest revision as of 00:03, 23 March 2023
| Hatefi Y, Haavik AG, Fowler LR, Griffiths DE (1962) Studies on the electron transfer system XLII. Reconstitution of the electron transfer system. J Biol Chem 237:2661-9. https://doi.org/10.1016/S0021-9258(19)73804-6 |
Hatefi Y, Haavik AG, Fowler LR, Griffiths DE (1962) J Biol Chem
Abstract: 1. It has been shown that the electron transfer system in beef heart mitochondria may be reconstituted either totally or in any desired sequential segment by appropriate combinations of two or more of the four primary complexes that have been isolated in highly purified form in this laboratory.
2. The four enzyme systems that collectively comprise the complete machinery for transfer of electrons from reduced diphosphopyridine nucleotide (DPNH; =NADH) and succinate to oxygen re: I, DPNH-coenzyme Q reductase; II, succinic-coenzyme Q reductase; III, QH2-cytochrome c reductase; and IV, cytochrome c reductase. The specific inhibitors of each complex have been studied.
3. By appropriate combinations of the primary complexes the following secondary activities have been reconstituted: V, DPNH-cytochrome c reductase; VI, succinic-cytochrome c reductase; VII, DPNH, succinic-cytochrome c reductase; VIII, DPNH oxidase; IX, succinic oxidase; and X, DPNH, succinic oxidase activity. The general oxidation-reduction properties of the reconstituted systems, both in the presence and the absence of the usual specific inhibitors of the electron transfer system, are essentially the same as those found for the same activities in the intact mitochondria and in the integrated particles derived therefrom.
4. The reconstituted activities are quite stable to repeated freezing, thawing, and storage at -2O °C, and for the most part, when once formed, are not dissociated by dilution of the mixture or by centrifugation. The evidence supporting the conclusion that reconstitution necessarily involves a particle-particle interaction is discussed. • Keywords: Electron transfer, DPNH-coenzyme Q reductase, Succinic-coenzyme Q reductase, QH2-cytochrome c reductase, Cytochrome c reductase, Beef heart
Electron transfer system versus electron transport chain
- More details: »Electron transfer-pathway
Cited by
- Gnaiger E (2020) Mitochondrial pathways and respiratory control. An introduction to OXPHOS analysis. 5th ed. Bioenerg Commun 2020.2. https://doi.org/10.26124/bec:2020-0002
Labels:
Organism: Bovines
Tissue;cell: Heart
Preparation: Isolated mitochondria
Enzyme: Complex II;succinate dehydrogenase, Complex IV;cytochrome c oxidase
Regulation: Substrate
Coupling state: ET
Made history, Electron transfer pathway, BEC 2020.2