Difference between revisions of "Prasuhn 2021 Front Cell Dev Biol"
(Created page with "{{Publication |title=Prasuhn J, Davis RL, Kumar KR (2021) Targeting mitochondrial impairment in Parkinson's disease: challenges and opportunities. Front Cell Dev Biol 8:615461...") |
|||
| Line 8: | Line 8: | ||
|editor=Gnaiger E | |editor=Gnaiger E | ||
}} | }} | ||
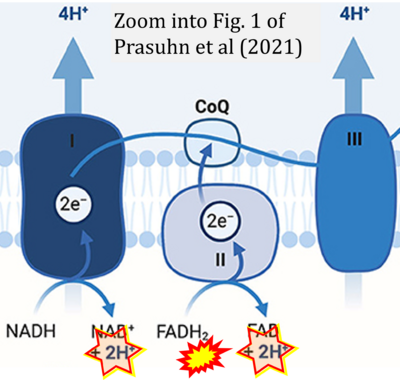
[[File:Prasuhn 2021 Front Cell Dev Biol CORRECTION.png|right|400px]] | |||
{{Template:Correction FADH2 and S-pathway}} | |||
{{Labeling | {{Labeling | ||
|enzymes=Complex II;succinate dehydrogenase | |enzymes=Complex II;succinate dehydrogenase | ||
}} | }} | ||
Revision as of 07:48, 15 April 2023
| Prasuhn J, Davis RL, Kumar KR (2021) Targeting mitochondrial impairment in Parkinson's disease: challenges and opportunities. Front Cell Dev Biol 8:615461. doi: 10.3389/fcell.2020.615461 |
Prasuhn J, Davis RL, Kumar KR (2021) Front Cell Dev Biol
Abstract: The underlying pathophysiology of Parkinson's disease is complex, but mitochondrial dysfunction has an established and prominent role. This is supported by an already large and rapidly growing body of evidence showing that the role of mitochondrial (dys)function is central and multifaceted. However, there are clear gaps in knowledge, including the dilemma of explaining why inherited mitochondriopathies do not usually present with parkinsonian symptoms. Many aspects of mitochondrial function are potential therapeutic targets, including reactive oxygen species production, mitophagy, mitochondrial biogenesis, mitochondrial dynamics and trafficking, mitochondrial metal ion homeostasis, sirtuins, and endoplasmic reticulum links with mitochondria. Potential therapeutic strategies may also incorporate exercise, microRNAs, mitochondrial transplantation, stem cell therapies, and photobiomodulation. Despite multiple studies adopting numerous treatment strategies, clinical trials to date have generally failed to show benefit. To overcome this hurdle, more accurate biomarkers of mitochondrial dysfunction are required to detect subtle beneficial effects. Furthermore, selecting study participants early in the disease course, studying them for suitable durations, and stratifying them according to genetic and neuroimaging findings may increase the likelihood of successful clinical trials. Moreover, treatments involving combined approaches will likely better address the complexity of mitochondrial dysfunction in Parkinson's disease. Therefore, selecting the right patients, at the right time, and using targeted combination treatments, may offer the best chance for development of an effective novel therapy targeting mitochondrial dysfunction in Parkinson's disease.
• Bioblast editor: Gnaiger E
Correction: FADH2 and Complex II
- FADH2 is shown as the substrate feeding electrons into Complex II (CII). This is wrong and requires correction - for details see Gnaiger (2024).
- Gnaiger E (2024) Complex II ambiguities ― FADH2 in the electron transfer system. J Biol Chem 300:105470. https://doi.org/10.1016/j.jbc.2023.105470 - »Bioblast link«
Labels:
Enzyme: Complex II;succinate dehydrogenase