Read 2021 Redox Biol: Difference between revisions
(Created page with "{{Publication |title=Read AD, Bentley RE, Archer SL, Dunham-Snary KJ (2021) Mitochondrial iron-sulfur clusters: Structure, function, and an emerging role in vascular biology....") |
No edit summary |
||
| Line 7: | Line 7: | ||
|abstract=Iron-sulfur (Fe-S) clusters are essential cofactors most commonly known for their role mediating electron transfer within the mitochondrial respiratory chain. The Fe-S cluster pathways that function within the respiratory complexes are highly conserved between bacteria and the mitochondria of eukaryotic cells. Within the electron transport chain, Fe-S clusters play a critical role in transporting electrons through Complexes I, II and III to cytochrome c, before subsequent transfer to molecular oxygen. Fe-S clusters are also among the binding sites of classical mitochondrial inhibitors, such as rotenone, and play an important role in the production of mitochondrial reactive oxygen species (ROS). Mitochondrial Fe-S clusters also play a critical role in the pathogenesis of disease. High levels of ROS produced at these sites can cause cell injury or death, however, when produced at low levels can serve as signaling molecules. For example, Ndufs2, a Complex I subunit containing an Fe-S center, N2, has recently been identified as a redox-sensitive oxygen sensor, mediating homeostatic oxygen-sensing in the pulmonary vasculature and carotid body. Fe-S clusters are emerging as transcriptionally-regulated mediators in disease and play a crucial role in normal physiology, offering potential new therapeutic targets for diseases including malaria, diabetes, and cancer. | |abstract=Iron-sulfur (Fe-S) clusters are essential cofactors most commonly known for their role mediating electron transfer within the mitochondrial respiratory chain. The Fe-S cluster pathways that function within the respiratory complexes are highly conserved between bacteria and the mitochondria of eukaryotic cells. Within the electron transport chain, Fe-S clusters play a critical role in transporting electrons through Complexes I, II and III to cytochrome c, before subsequent transfer to molecular oxygen. Fe-S clusters are also among the binding sites of classical mitochondrial inhibitors, such as rotenone, and play an important role in the production of mitochondrial reactive oxygen species (ROS). Mitochondrial Fe-S clusters also play a critical role in the pathogenesis of disease. High levels of ROS produced at these sites can cause cell injury or death, however, when produced at low levels can serve as signaling molecules. For example, Ndufs2, a Complex I subunit containing an Fe-S center, N2, has recently been identified as a redox-sensitive oxygen sensor, mediating homeostatic oxygen-sensing in the pulmonary vasculature and carotid body. Fe-S clusters are emerging as transcriptionally-regulated mediators in disease and play a crucial role in normal physiology, offering potential new therapeutic targets for diseases including malaria, diabetes, and cancer. | ||
}} | }} | ||
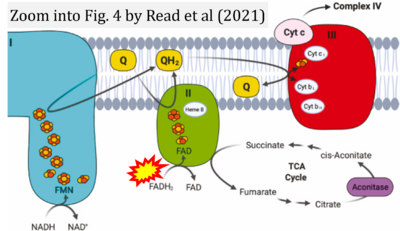
[[File:Read 2021 Redox Biol CORRECTION.png|right|400px]] | |||
{{Template:Correction FADH2 and S-pathway}} | |||
{{Labeling | {{Labeling | ||
|enzymes=Complex I, Complex II;succinate dehydrogenase, Complex III | |enzymes=Complex I, Complex II;succinate dehydrogenase, Complex III | ||
}} | }} | ||
Latest revision as of 17:59, 2 April 2023
» [[Has info::PMID: 34656823 Open Access]]
Was written by::Read AD, Was written by::Bentley RE, Was written by::Archer SL, Was written by::Dunham-Snary KJ (Was published in year::2021) Was published in journal::Redox Biol
Abstract: [[has abstract::Iron-sulfur (Fe-S) clusters are essential cofactors most commonly known for their role mediating electron transfer within the mitochondrial respiratory chain. The Fe-S cluster pathways that function within the respiratory complexes are highly conserved between bacteria and the mitochondria of eukaryotic cells. Within the electron transport chain, Fe-S clusters play a critical role in transporting electrons through Complexes I, II and III to cytochrome c, before subsequent transfer to molecular oxygen. Fe-S clusters are also among the binding sites of classical mitochondrial inhibitors, such as rotenone, and play an important role in the production of mitochondrial reactive oxygen species (ROS). Mitochondrial Fe-S clusters also play a critical role in the pathogenesis of disease. High levels of ROS produced at these sites can cause cell injury or death, however, when produced at low levels can serve as signaling molecules. For example, Ndufs2, a Complex I subunit containing an Fe-S center, N2, has recently been identified as a redox-sensitive oxygen sensor, mediating homeostatic oxygen-sensing in the pulmonary vasculature and carotid body. Fe-S clusters are emerging as transcriptionally-regulated mediators in disease and play a crucial role in normal physiology, offering potential new therapeutic targets for diseases including malaria, diabetes, and cancer.]]
Correction: FADH2 and Complex II
- FADH2 is shown as the substrate feeding electrons into Complex II (CII). This is wrong and requires correction - for details see Gnaiger (2024).
- Gnaiger E (2024) Complex II ambiguities ― FADH2 in the electron transfer system. J Biol Chem 300:105470. https://doi.org/10.1016/j.jbc.2023.105470 - »Bioblast link«
Labels:
Enzyme: Enzyme::Complex I, Enzyme::Complex II;succinate dehydrogenase, Enzyme::Complex III