Sanchez 2001 Br J Pharmacol
| Sanchez H, Zoll J, Bigard X, Veksler V, Mettauer B, Lampert E, Lonsdorfer J, Ventura-Clapier R (2001) Effect of cyclosporin A and its vehicle on cardiac and skeletal muscle mitochondria: relationship to efficacy of the respiratory chain. Br J Pharmacol 133:781-8. https://doi.org/10.1038/sj.bjp.0704129 |
Sanchez H, Zoll J, Bigard X, Veksler V, Mettauer B, Lampert E, Lonsdorfer J, Ventura-Clapier R (2001) Br J Pharmacol
Abstract: Although cyclosporin (CsA) is considered to be the best immunosuppressive molecule in transplantation, it has been suspected to alter mitochondrial respiration of various tissues.
We evaluated the acute effect of CsA and its vehicle on maximal oxidative capacity (Vmax) of cardiac, soleus and gastrocnemius muscles of rats by an oxygraphic method in saponin skinned muscle fibres. The effects of Sandimmun (a formulation of CsA), vehicle of Sandimmun (cremophor and ethanol (EtOH)), CsA in EtOH and EtOH alone were tested. Increasing concentrations (5 – 20 – 50 – 100 μM) of CsA (or vehicles) were used.
Sandimmun profoundly altered the Vmax of all muscles. For example, at 20 μM, inhibition reached 18±3, 23±5, 45±5%, for heart, soleus and gastrocnemius respectively. There were only minor effects of CsA diluted in EtOH and EtOH alone on Vmax of cardiac muscle. Because the effects of vehicle on Vmax were similar or higher than those of Sandimmun, the inhibition of oxidative capacity could be entirely attributed to the vehicle for all muscles.
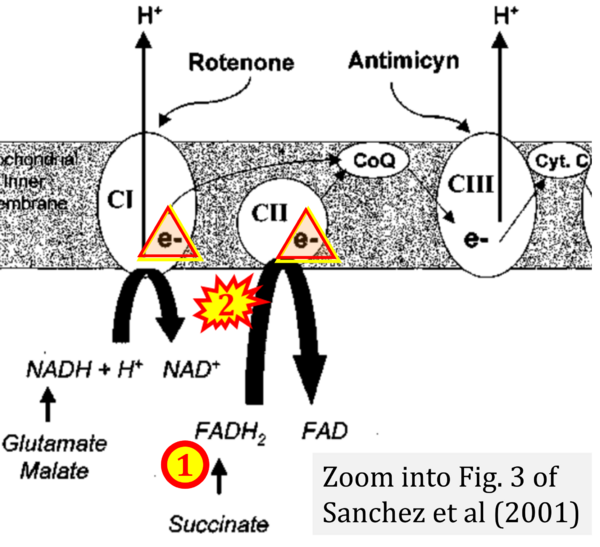
Next, we investigated the potential sites of action of the vehicle on the different complexes of the mitochondrial respiratory chain by using specific substrates and inhibitors. The vehicle affected mitochondrial respiration mainly at the level of complex I (≈−85 % in skeletal muscles, and −32 % in heart), but also at complex IV (≈−26 % for all muscles).
The mechanism of action of the vehicle on the mitochondrial membrane and the implications for the clinical use of immunosuppressive drugs are discussed.
• Bioblast editor: Gnaiger E
Correction: FADH2 and Complex II
- FADH2 is shown as the substrate feeding electrons into Complex II (CII). This is wrong and requires correction - for details see Gnaiger (2024).
- Gnaiger E (2024) Complex II ambiguities ― FADH2 in the electron transfer system. J Biol Chem 300:105470. https://doi.org/10.1016/j.jbc.2023.105470 - »Bioblast link«
Labels: MiParea: Respiration, Pharmacology;toxicology