Talk:TMRM
TMRM with the O2k-Fluorescence LED2-Module
TMRM with the O2k-Fluorescence LED2-Module: Exploratory Experiment
- 100 mM potassium phosphate buffer, T= 37°C
- Fluorescence-Sensor Green, Filter Set AmR, light intensity: Amp Polarization voltage = 100, Amp Gain = 1000
- TMRM stock: 100 µM in DMSO
- titration TIP: 5x 0.2 µl (5x10 nM), 5 x 1 µl (5 x 50 nM), 7 x 2 µl (7 x 100 nM) interval: 120 s
TMRM with the O2k-Fluorescence LED2-Module: Experiments with mouse liver mitochondria by C. Chinopoulos
Mouse liver mitochondria
Protein (BCA assay) 130.5 mg/ml
O2k chamber: 0.5 mg/ml (0.489 mg/ml)
Experimental buffer composition:
KCl 8 mM, K-gluconate 110 mM, Mannitol 10 mM, NaCl 10 mM, Hepes (free acid) 10 mM, K2HPO4 10 mM, K-EGTA 0.01 mM, BSA 0.5 mg/ml, pH 7.25 (KOH), MgCl2 1 mM (added in the buffer)
Glutamate: 10 mM (40 µl of a 1 M stock)
Malate: 2 mM (16 µl of a 0.5 M stock)
Pyruvate: 5 mM (20 µl of a 1 M stock)
Carboxyatractyloside (cATR): 0.5 µM 2 µl of a (1 mM stock)
SF6847: 0.5 µM (2 µl of a 1 mM stock)
ADP: 2 mM (40 µl of a 200 mM stock)
Experimental conditions:
4ml chamber volume
Fluorescence-Sensor Green, Filter Set AmR, light intensity: Amp Polarization voltage = 1000 (equivalent to level setting "6" on the Fluorescence module Series A front panel) , gain 1000
T = 37.0 °C
TMRM was added 20-30 sec before addition of mitos to the Oxygraph chamber
Figure legends:
Concurrent measurement of respiration and mitochondrial membrane potential (mtMP) in mouse liver mitochondria (MLM): 0.5 mg/ml MLM were added to the O2k chamber with 4 ml experimental buffer (composition see above) including pyruvate, glutamate and malate and containing TMRM at variable concentrations as indicated. After 100 s 2 mM ADP was added, followed by 0.5 mM CATR at 350 s and 0.5 µM SF6847 at 450 s. The concentration of the uncoupler SF6847 used was twice the concentration required to induce maximum oxygen flux so as to ensure maximum depolarization of mtMP.
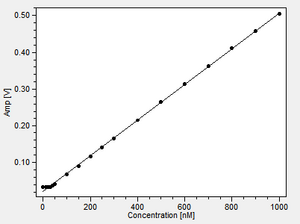
Analysis of respiratory rates indicated that neither LEAK nor OXPHOS rates were significantly affected by TMRM up to 4000 nM, with an RCR of at least 15 in each case. At 5000 nM TMRM LEAK appeared to be elevated and RCR accordingly diminished to a value of 12.
Please note the very different scaling for the fluorescence signal in the individual graphs! The range is always described by the ∆=XY at the end of the legend.
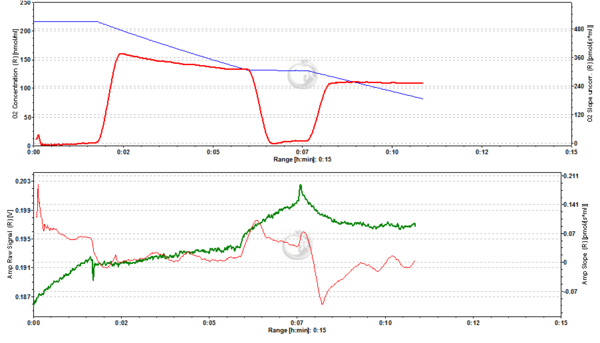
Figure 1. Zero nM TMRM. Control measurement of MLM in the absence of TMRM showing control respiratory rates (A; blue line: oxygen concentration, red line: oxygen flux) and background fluorescence (B; green line: raw signal of fluorescence, red line: rate of change in fluorescence). The maximum change in fluorescence induced by addition of chemicals amounts to approx. 0.003 fluorescence units (FU) (∆=0.003 FU).
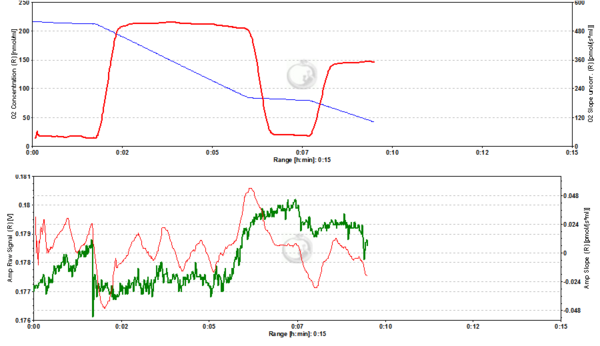
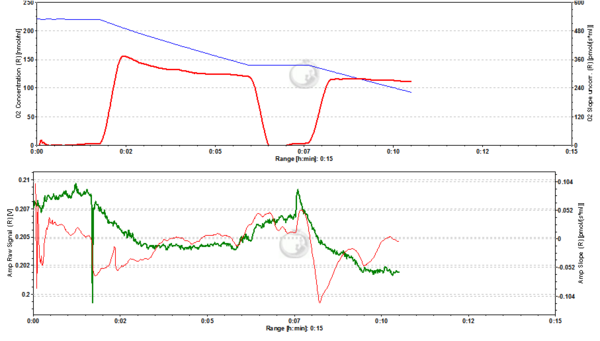
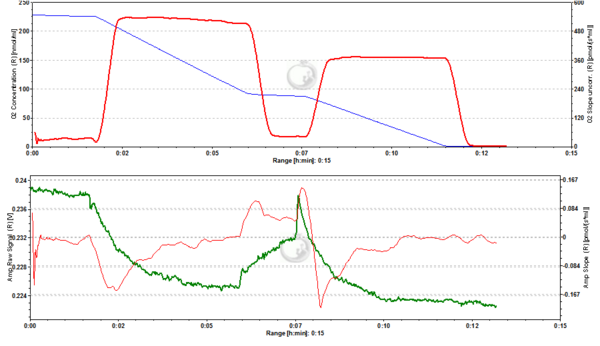
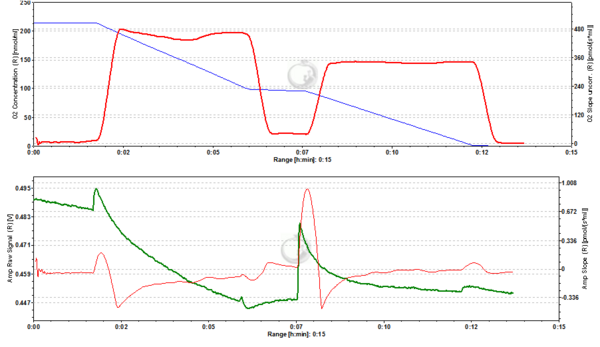
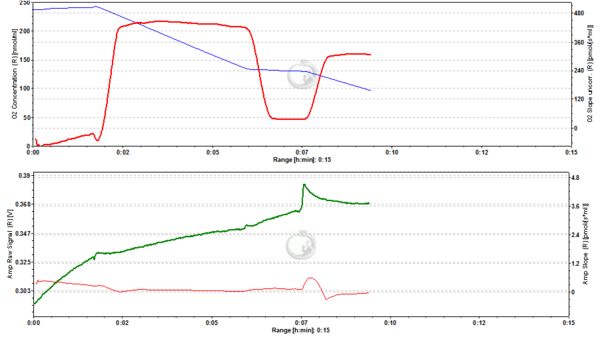
Figure2. 20 nM TMRM. MLM incubated with 20 nM TMRM and energized by CI-linked substrates display a basal level of fluorescence due to accumulation of the dye according to their mtMP in the LEAK state. Upon addition of ADP proton motive force is partially dissipated to drive the generation of ATP (OXPHOS state) and this leads to a decrease of mtMP. A decrease of mtMP causes the release of accumulated TMRM and this is reflected by a decrease of the fluorescence signal. Addition of the inhibitor of the adenine nucleotide translocator (ANT) carboxyatractyloside (cATR) inhibits ATP synthesis (LEAK in the presence of ATP) and thus the influx of H+ through the ATP synthase (in addition to the fact that cATR stops the electrogenic exchange of ATP4- for ADP3-, thus sparing the loss of a negative charge per each exchange). The consecutive slow restoration of mtMP causes a re-accumulation of TMRM and hence an increase of the fluorescence signal. Finally, upon addition of the uncoupler SF6847 a collapse of mtMP is observed and thus a release of TMRM with an associated decrease of fluorescence. The short-lived peak of fluorescence after addition of uncoupler is due to transient unquenching of matrix TMRM caused by the rapid release of the dye. ∆=0.008 FU.
Figure 3. 50 nM TMRM. MLM incubated with 50 nM TMRM, other conditions as in Fig. 2. ∆=0.016 FU.
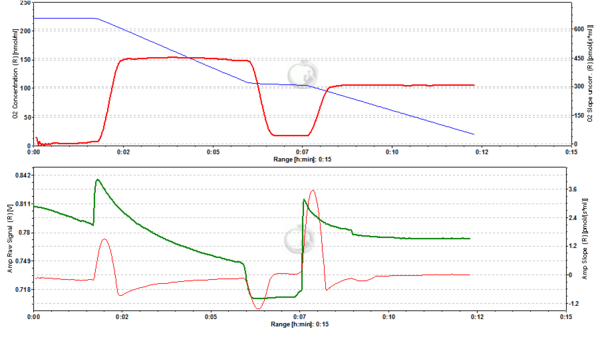
Figure 4. 200 nM TMRM. MLM incubated with 200 nM TMRM, other conditions as in Fig. 2. Short-lived peaks of fluorescence observed after addition of chemicals are due to transient unquenching of matrix TMRM caused by the rapid release of the dye. ∆=0.100 FU.
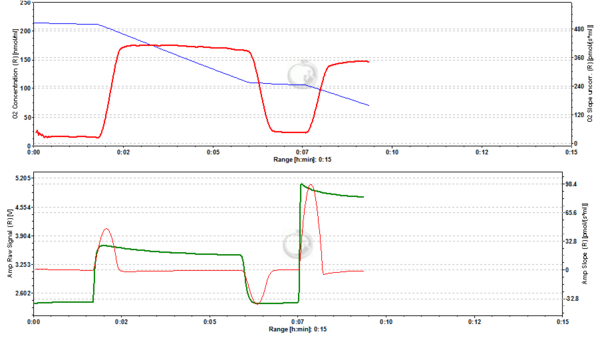
Figure 5. 500 nM TMRM. MLM incubated with 500 nM TMRM, other conditions as in Fig. 2. At this concentration TMRM is at the verge of alternating between a response in quenching and unquenching mode. In the latter mode the relation between mtMP and TMRM concentration in the matrix becomes inverted in that a decrease of mtMP leads to an increase of fluorescence due to unquenching of fluorescence upon release of the dye. ∆=0.16 FU.
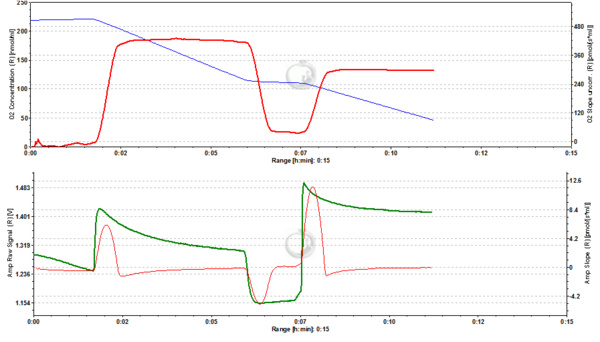
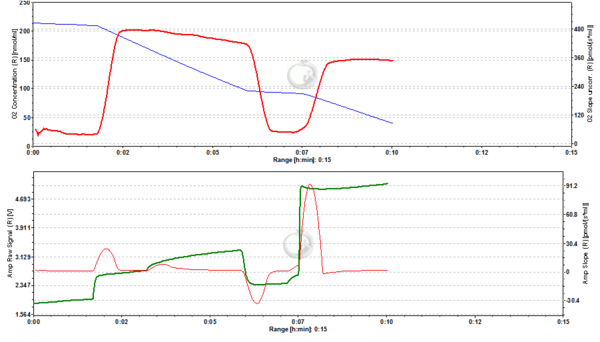
Figure 6. 1000 nM TMRM. MLM incubated with 1000 nM TMRM, other conditions as in Fig. 2. In the unquenching mode the decrease of mtMP resulting from addition of ADP is mirrored by an increase of fluorescence, while the increase of mtMP due to inhibition of ATP synthesis is seen as a decrease of fluorescence. The collapse of mtMP is again associated with an increased fluorescence signal. ∆=0.295 FU.
Figure 7. 2000 nM TMRM. MLM incubated with 2000 nM TMRM, other conditions as in Fig. 2, for explanation of changes in mtMP and fluorescence see legend to Fig. 6. ∆=0.956 FU.
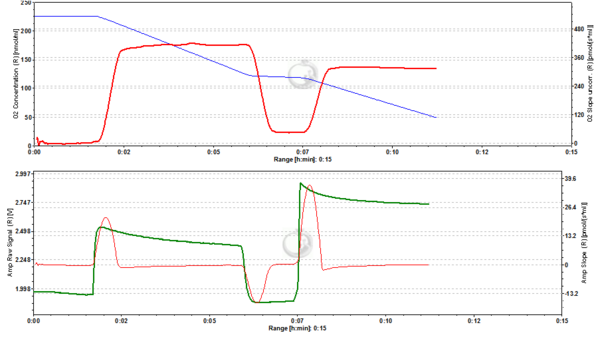
Figure 8. 5000 nM TMRM. MLM incubated with 5000 nM TMRM, other conditions as in Fig. 2. , for explanation of changes in mtMP and fluorescence see legend to Fig. 6. The instability of the signal in the presence of ADP may reflect the onset of toxicity at this concentration of TMRM, possibly resulting in the opening of the permeability transition pore of a sub-population of mitochondria. ∆=2.601 FU.
Figure 9. 4000 nM TMRM. MLM incubated with 4000 nM TMRM, other conditions as in Fig. 2, for explanation of changes in mtMP and fluorescence see legend to Fig. 6. ∆=2.535 FU.
Figure 10. 0 nM TMRM. Control measurement in the absence of TMRM. Conditions as in Fig. 1. ∆=0.070 FU.
Figure 11. 0 nM TMRM. Control measurement in the absence of TMRM. Conditions as in Fig. 1. ∆=0.015 FU.
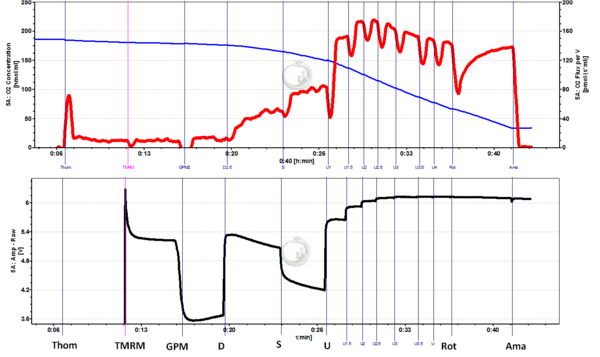
TMRM with the O2k-Fluorescence LED2-Module: Experiments with rat liver homogenate by G. Krumschnabel and Z. Sumbalova
Wistar rat liver tissue homogenate
1 mg Fw/ml
Experimental buffer: MiR05
Glutamate: 10 mM; Pyruvate: 5 mM; Malate: 2 mM; ADP: 2.5 mM; Succinate: 10 mM; CCCP (U) added in 0.5 µM steps; Rotenone 1 µg/ml; Antimycin A 2.5µM:
Experimental conditions: 2 ml chamber volume, Fluorescence-Sensor Green, Filter Set AmR, light intensity: Amp Polarization voltage = 1000 (equivalent to level setting "6" on the Fluorescence module Series A front panel), gain 500, T = 37.0 °C
Figure legends: Concurrent measurement of respiration [upper panel; pmol/(s*ml)] and mitochondrial membrane potential (mtMP) (lower panel; arbitrary units) in tissue homogenate (Thom) from mouse liver: 1 mg/ml Thom was added to the O2k chamber with 2 ml experimental buffer and then 2 µM TMRM were added as indicated. This was followed by addition of glutamate, pyruvate and malate to induce LEAK respiration. Next, 2.5 mM ADP was added to elicit CI-linked OXPHOS, then succinate was injected to obtain CI&II-linked OXPHOS, before titrating uncoupler CCCP (U) in 0.5 µM steps to induce fully uncoupled respiration and fully collapsed mtMP. It should be noted that CCCP concentration required for complete collapse of mtMP was higher that needed for maximum respiration and that respiration was already inhibited at this higher CCCP. Finally, CI-inhibitor rotenone (Rot) and CIII-inhibitor antimycin A (Ama) were added to obtain residual oxygen consumption (ROX), which however did not further affect mtMP.