Cooper 2000 Sunderland (MA): Sinauer Associates
| Cooper GM (2000) The cell: a molecular approach. 2nd edition. Sunderland (MA): Sinauer Associates Available from: https://www.ncbi.nlm.nih.gov/books/NBK9885/ |
Cooper GM (2000) Sunderland (MA): Sinauer Associates
Abstract: As in the first edition, The Cell is focused on the molecular biology of cells as a unifying theme, with specialized topics discussed throughout the book as examples of more general principles. Aspects of developmental biology, the immune system, the nervous system, and plant biology are thus discussed in their broader biological context in chapters covering areas such as genome structure, gene expression, DNA rearrangements, the plasma membrane, cell signaling, and the cell cycle. Relationships between cell biology and medicine are similarly discussed throughout the text, as well as being highlighted in the Molecular Medicine essays that are included as a special feature in each chapter. These discussions illustrate the striking impact of molecular and cellular biology on human health, and are intended to stimulate as well as inform those students interested in medicine.
• Bioblast editor: Gnaiger E
Complex II ambiguities - selected qotes from Chapter 10
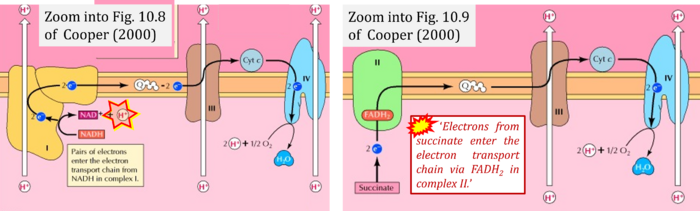
- (1) 'Electrons from NADH enter the electron transport chain in complex I, .. A distinct protein complex (complex II), which consists of four polypeptides, receives electrons from the citric acid cycle intermediate, succinate (Figure 10.9). These electrons are transferred to FADH2, rather than to NADH, and then to coenzyme Q.'
- Comment: Here, the frequent comparison is made between FADH2 (linked to CII) and NADH (linked to CI).
- (2) 'In contrast to the transfer of electrons from NADH to coenzyme Q at complex I, the transfer of electrons from FADH2 to coenzyme Q is not associated with a significant decrease in free energy and, therefore, is not coupled to ATP synthesis.'
- Comment: Note that CI is in the path of the transfer of electrons from NADH to coenzyme Q. In contrast, the transfer of electrons from FADH2 to coenzyme Q is downstream of CII. Thus even a large Gibbs force ('decrease in free energy') in FADH2→Q would fail to drive the coupled process of proton translocation through CII, since the Gibbs force in S→FADH2 is missing. (In parentheses: None of these steps are coupled to ATP synthesis. Redox-driven proton translocation should not be confused with pmF-driven phosphorylation of ADP).
- (2) 'In contrast to the transfer of electrons from NADH to coenzyme Q at complex I, the transfer of electrons from FADH2 to coenzyme Q is not associated with a significant decrease in free energy and, therefore, is not coupled to ATP synthesis.'
- (3) 'Electrons from succinate enter the electron transport chain via FADH2 in complex II. They are then transferred to coenzyme Q and carried through the rest of the electron transport chain ..'
- Comment: The ambiguity - CII receives electrons (1) from succinate, yet it is suggested that electrons (from succinate) enter the electron transport chain (3) via FADH2 in complex II. Then two contrasting definitions are implied of the term 'electron transport chain' or better membrane-bound electron transfer system, membrane-ETS. (a) If CII is part of the membrane-ETS, then electrons enter the membrane-ETS from succinate (1) but not from FADH2. (b) If electrons enter the 'electron transport chain' via FADH2 in complex II (3), then CII would be upstream and hence not part of the membrane-ETS (to which conclusion, obviously - see Figure - nobody would agree). Dismissing concept (b) of the membrane-ETS, then remains the ambiguity, if electrons enter the membrane-ETS from FADH2 (3, wrong) or from succinate (1, correct).
Correction: FADH2 and Complex II
- FADH2 is shown as the substrate feeding electrons into Complex II (CII). This is wrong and requires correction - for details see Gnaiger (2024).
- Gnaiger E (2024) Complex II ambiguities ― FADH2 in the electron transfer system. J Biol Chem 300:105470. https://doi.org/10.1016/j.jbc.2023.105470 - »Bioblast link«
Hydrogen ion ambiguities in the electron transfer system
Communicated by Gnaiger E (2023-10-08) last update 2023-11-10
- Electron (e-) transfer linked to hydrogen ion (hydron; H+) transfer is a fundamental concept in the field of bioenergetics, critical for understanding redox-coupled energy transformations.
- However, the current literature contains inconsistencies regarding H+ formation on the negative side of bioenergetic membranes, such as the matrix side of the mitochondrial inner membrane, when NADH is oxidized during oxidative phosphorylation (OXPHOS). Ambiguities arise when examining the oxidation of NADH by respiratory Complex I or succinate by Complex II.
- Oxidation of NADH or succinate involves a two-electron transfer of 2{H++e-} to FMN or FAD, respectively. Figures indicating a single electron e- transferred from NADH or succinate lack accuracy.
- The oxidized NAD+ is distinguished from NAD indicating nicotinamide adenine dinucleotide independent of oxidation state.
- NADH + H+ → NAD+ +2{H++e-} is the oxidation half-reaction in this H+-linked electron transfer represented as 2{H++e-} (Gnaiger 2023). Putative H+ formation shown as NADH → NAD+ + H+ conflicts with chemiosmotic coupling stoichiometries between H+ translocation across the coupling membrane and electron transfer to oxygen. Ensuring clarity in this complex field is imperative to tackle the apparent ambiguity crisis and prevent confusion, particularly in light of the increasing number of interdisciplinary publications on bioenergetics concerning diagnostic and clinical applications of OXPHOS analysis.



