Willson 2022 Blood
| Willson JA, Arienti S, Sadiku P, Reyes L, Coelho P, Morrison T, Rinaldi G, Dockrell DH, Whyte MKB, Walmsley SR (2022) Neutrophil HIF-1α stabilization is augmented by mitochondrial ROS produced via the glycerol 3-phosphate shuttle. Blood 139:281-6. doi: 10.1182/blood.2021011010 |
Willson JA, Arienti S, Sadiku P, Reyes L, Coelho P, Morrison T, Rinaldi G, Dockrell DH, Whyte MKB, Walmsley SR (2022) Blood
Abstract: Neutrophils are predominantly glycolytic cells that derive little ATP from oxidative phosphorylation; however, they possess an extensive mitochondrial network and maintain a mitochondrial membrane potential. Although studies have shown neutrophils need their mitochondria to undergo apoptosis and regulate NETosis, the metabolic role of the respiratory chain in these highly glycolytic cells is still unclear. Recent studies have expanded on the role of reactive oxygen species (ROS) released from the mitochondria as intracellular signaling molecules. Our study shows that neutrophils can use their mitochondria to generate ROS and that mitochondrial ROS release is increased in hypoxic conditions. This is needed for the stabilization of a high level of the critical hypoxic response factor and pro-survival protein HIF-1α in hypoxia. Further, we demonstrate that neutrophils use the glycerol 3-phosphate pathway as a way of directly regulating mitochondrial function through glycolysis, specifically to maintain polarized mitochondria and produce ROS. This illustrates an additional pathway by which neutrophils can regulate HIF-1α stability and will therefore be an important consideration when looking for treatments of inflammatory conditions in which HIF-1α activation and neutrophil persistence at the site of inflammation are linked to disease severity.
• Bioblast editor: Gnaiger E
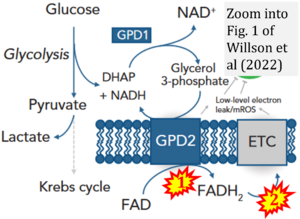
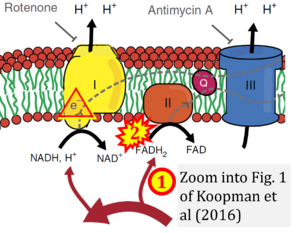
- Comment (Cardoso Luiza, Gnaiger Erich, 2023-08-05): Figure 1 shows FADH2 to be formed in the mitochondrial matrix from (1) GPD2 (CGpDH), feeding electrons further (2) into the 'ETC' (ETS). Combined with FADH2 formed from (1) the TCA cycle and feeding (2) into CII (Fig. 1 on the right; one of >120 examples discussed as CII-ambiguities), one may arrive at the erroneous conclusion on a direct role of CII in the oxidation of glycerol-3-phosphate, analogous to false representations of CII involved in fatty acid oxidation.
Correction: FADH2 and Complex II
- FADH2 is shown as the substrate feeding electrons into Complex II (CII). This is wrong and requires correction - for details see Gnaiger (2024).
- Gnaiger E (2024) Complex II ambiguities ― FADH2 in the electron transfer system. J Biol Chem 300:105470. https://doi.org/10.1016/j.jbc.2023.105470 - »Bioblast link«
Labels: