Difference between revisions of "Talk:Magnesium Green"
(Created page with "== Mg green concentrations== used by OROBOROS for testing: stock: 2 mM in chamber: 2 µM") |
|||
| Line 3: | Line 3: | ||
stock: 2 mM | stock: 2 mM | ||
in chamber: 2 µM | in chamber: 2 µM | ||
== Mg2+ Calibration experiment == | |||
The following calibration experiment was performed with a medium contaminated with Ca2+. Ca2+ impurities are a particular problem because a.) Mg green binds stronger to Ca2+ than to Mg2+ and b.) Ca2+ impurities are very common. The Ca2+ impurities were complexed by an EGTA titration before the Mg2+ calibration, without (in this case) negative effects on the Mg2+ performance. | |||
* O2k with [[O2k-Fluo LED2-Module]], [[Fluorescence-Sensor Blue]], and [[Filter Set MgG / CaG]] | |||
* LED intensity: Amp polarisation voltage = 100 mV, Amp Gain = 1000, data sampling interval = 2 s, 37°C | |||
* medium: ANT buffer as desriebd by Christo Chiopoulos | |||
Titrations: | |||
* Mg green 2 µL 2 mM --> 2 µM | |||
* EGTA titration: 3 * (5 µl 20 mM --> 50 µM) until very small changes in signal | |||
* DTPA 2 µl 20 mM -->20 µM | |||
* 10 * ( 4 µl MgCl2 0.1 M --> 0.2 mM) | |||
* EDTA 16 µl 500 mM --> 4 mM | |||
* EDTA 2 µl 500 mM --> 0.5 mM: no further change | |||
* MgCl2 80 µl 1 M --> 40 mM | |||
* MgCl2 20 µl 1 M --> 10 mM: no further change | |||
(To allow performance tests a substance mimicking light scattering of typical samples was added to the medium, however this is not relevant for the results presented here.) | |||
The addition of a very small concentration of either EDTA or DTPA in the beginning of the experiment is suggested by the produced of the fluorophores (for all similar fluorophores) to remove traces of transition metal cations. | |||
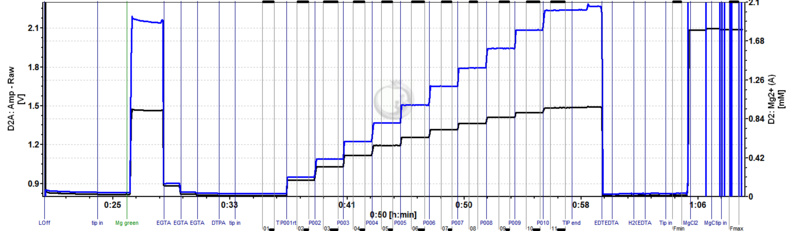
Shown in the DatLab graph is the original raw voltage as recoded by the fluorescence sensor and the Mg2+ concentration. For calibration determining KD and slope) and plotting the calculated Mg concentration (via the experimental scripting function of DatLab) the following relation was used | |||
[Mg2+] = Kd * ( F- Fmin) / (Fmac -F) + intercept | |||
with | |||
F .......Fluorescence signal | |||
Fmin..... minimal fluorescence signal, free Mg2+ concentration = 0, established by excess EDTA | |||
Fmax .....maximal fluorescence signal, fluorophore indicator saturated with Mg2+, established by excess of MgCl2 | |||
[[File:MF686b A module DL.png|800px]] | |||
[[File:MF686b_A_module_calibration.png|400px]] | |||
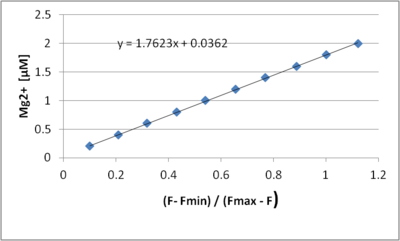
The determined KD value (1.8 mM) is influenced by the presence of both Ca2+ and EGTA. However, by using the the determined KD value the linearity of the claulated Mg2+ concnetraion is still very good. Both free AGTA and free Ca2+ concetrations that are significatyl higher than the smallest Mg2 but significatyl lower tahn the higher Mg2 concetraion will however affect the apparent linearity. So a as ca free medium as posisble shoule be used a a mtter of principle, even if this example sjows that good results can be be otained even in the presecenof have Ca conatmination. | |||
[[User:Fasching Mario|Fasching Mario]] 12:02, 16 March 2015 (CET) | |||
[[File:MF686b_A_module_calibration.png | |||
Revision as of 13:02, 16 March 2015
Mg green concentrations
used by OROBOROS for testing: stock: 2 mM in chamber: 2 µM
Mg2+ Calibration experiment
The following calibration experiment was performed with a medium contaminated with Ca2+. Ca2+ impurities are a particular problem because a.) Mg green binds stronger to Ca2+ than to Mg2+ and b.) Ca2+ impurities are very common. The Ca2+ impurities were complexed by an EGTA titration before the Mg2+ calibration, without (in this case) negative effects on the Mg2+ performance.
- O2k with O2k-Fluo LED2-Module, Fluorescence-Sensor Blue, and Filter Set MgG / CaG
- LED intensity: Amp polarisation voltage = 100 mV, Amp Gain = 1000, data sampling interval = 2 s, 37°C
- medium: ANT buffer as desriebd by Christo Chiopoulos
Titrations:
- Mg green 2 µL 2 mM --> 2 µM
- EGTA titration: 3 * (5 µl 20 mM --> 50 µM) until very small changes in signal
- DTPA 2 µl 20 mM -->20 µM
- 10 * ( 4 µl MgCl2 0.1 M --> 0.2 mM)
- EDTA 16 µl 500 mM --> 4 mM
- EDTA 2 µl 500 mM --> 0.5 mM: no further change
- MgCl2 80 µl 1 M --> 40 mM
- MgCl2 20 µl 1 M --> 10 mM: no further change
(To allow performance tests a substance mimicking light scattering of typical samples was added to the medium, however this is not relevant for the results presented here.)
The addition of a very small concentration of either EDTA or DTPA in the beginning of the experiment is suggested by the produced of the fluorophores (for all similar fluorophores) to remove traces of transition metal cations.
Shown in the DatLab graph is the original raw voltage as recoded by the fluorescence sensor and the Mg2+ concentration. For calibration determining KD and slope) and plotting the calculated Mg concentration (via the experimental scripting function of DatLab) the following relation was used
[Mg2+] = Kd * ( F- Fmin) / (Fmac -F) + intercept
with
F .......Fluorescence signal Fmin..... minimal fluorescence signal, free Mg2+ concentration = 0, established by excess EDTA Fmax .....maximal fluorescence signal, fluorophore indicator saturated with Mg2+, established by excess of MgCl2
The determined KD value (1.8 mM) is influenced by the presence of both Ca2+ and EGTA. However, by using the the determined KD value the linearity of the claulated Mg2+ concnetraion is still very good. Both free AGTA and free Ca2+ concetrations that are significatyl higher than the smallest Mg2 but significatyl lower tahn the higher Mg2 concetraion will however affect the apparent linearity. So a as ca free medium as posisble shoule be used a a mtter of principle, even if this example sjows that good results can be be otained even in the presecenof have Ca conatmination.
Fasching Mario 12:02, 16 March 2015 (CET)
[[File:MF686b_A_module_calibration.png