Description
Magnesium Green (MgG) is an extrinsic fluorophore that fluoresces when bound to Mg2+ and is used for measuring mitochondrial ATP production by mitochondrial preparations. Determination of mitochondrial ATP production is based on the different dissociation constants of Mg2+ for ADP and ATP, and the exchange of one ATP for one ADP across the mitochondrial inner membrane by the adenine nucleotide translocase (ANT). Using the dissociation constants for ADP-Mg2+ and ATP-Mg2+ and initial concentrations of ADP, ATP and Mg2+, the change in ATP concentration in the medium is calculated, which reflects mitochondrial ATP production.
Abbreviation: MgG
Reference: Chinopoulos 2014 Methods Enzymol, Cardoso 2021 BEC MgG
MitoPedia methods:
Fluorometry
MitoPedia O2k and high-resolution respirometry:
O2k-Open Support
Magnesium Green in high-resolution respirometry (HRR)
Instrument
- In high-resolution respirometry the MgG method is used with the O2k-FluoRespirometer with O2k-Fluo Smart-Module or O2k-Fluo LED2-Module selecting the Fluorescence-Sensor Blue/Filter Set MgG / CaG. Please see these pages for specific aspects of fluorescence measurements using the O2k.
- Use black stoppers with black cover-slips to exclude disturbances by external light sources.
- Before the experiments, switch off the illumination of the chambers (in [Oroboros O2k] \ [ O2k control ], or [F7]).
- Set the Gain for Amp sensor to 1000 and light intensity (Amp polarization voltage [mV]) to 500. These values can be modified by the user if needed (e.g., if a different MgG concentration is used).
The fluorescent dye
- Magnesium GreenTM is a registered trademark and available from Thermo Fisher Scientific (formerly: Invitrogen) in several formulations. For measuring mitochondrial ATP production a membrane impermeant formulation must be chosen (e.g. #M3733).
- The technique is based on detecting the exchange of ADP/ATP by ANT. Therefore, a membrane-permeant MgG should not be used, since it might diffuse to the mitochondrial intermembrane space and matrix.
Preparation of MgG solution
- Magnesium Green from Thermo Fischer Scientific (former Invitrogen): M3733 (Magnesium Green™, Pentapotassium Salt, cell impermeant); 1 mg vial, store at -20°C.
- Preparation of 1.1 mM stock solution (dissolved in H2O):
- Dissolve the complete vial of MgG (1 mg) in 992.6 µL of deionized H2O.
- Divide into 40 µL portions into 0.2 mL Eppendorf tubes (protect from light, use dark tubes preferably).
- Store frozen at -20 °C protected from light.
- Preparation of 1.1 mM stock solution (dissolved in H2O):
Final MgG concentration in the chamber
- Titration into 2 mL O2k-Chamber: 2 µL of 1.1 mM MgG stock solution, final concentration of 1.1 µM MgG.
Preparation of other solutions
MgCl2 solution
- Recommendation: MgCl2 solution from Sigma: M1028, 1 M solution. Prepare aliquots before use.
- The 1 M stock solution is used for addition at the beginning of an experiment (SUIT protocol).
- For calibration, a 0.1 M solution is needed.
- Preparation of 0.1 M stock solution (dissolved in H2O):
- This solution will be used for the calibration.
- Add 900 µL of deionized H2O in an eppendorf tube.
- Add 100 µL of the MgCl2 1M solution and mix.
ADP solution
- ADP from Merck (former Calbiochem): 117105 (Adenosine 5ʹ-Diphosphate, Potassium Salt), store at -20°C.
- Preparation of 0.2 M stock solution (dissolved in H2O):
- Weigh 1.0026 g, dilute in H2O
- Adjust pH to 6.9 with KOH, preferably on ice (with pHmeter calibrated on the same condition).
- Complete with H2O to 10 mL
- Aliquot (200 µL) and store at -20°C.
- Avoid thawing and re-freezing the aliquots.
- The concentration can be corrected by measuring the absorbance at 260 nm and using an extinction coefficient factor of εM = 15400 M-1⋅cm-1
- ADP should be prepared without any Mg2+ salt for the MgG technique!
- Preparation of 0.2 M stock solution (dissolved in H2O):
ATP solution
- ATP from Merck (former Sigma-Aldrich): A26209 (Adenosine 5′-triphosphate disodium salt hydrate), store at -20°C.
- Preparation of 0.2 M stock solution (dissolved in H2O):
- Weigh 1.1023 g, dilute in H2O
- Adjust pH to 6.9 with KOH, preferably on ice (with pHmeter calibrated on the same condition). Avoid adding too much KOH as higher pH may favor the hydrolysis of the ATP.
- Complete with H2O to 10 mL
- Aliquot (200 µL) and store at -20°C.
- Avoid thawing and re-freezing the aliquots.
- The concentration can be corrected by measuring the absorbance at 260 nm and using an extinction coefficient factor of εM = 15400 M-1⋅cm-1
- Preparation of 0.2 M stock solution (dissolved in H2O):
Experimental media for the MgG assay
- This method is not suitable for buffers containing high concentrations of Mg2+. It has been used in the Oroboros Laboratories with a modified formulation of MiR05 with 1 mM MgCl2 instead of the original 3 mM MgCl2. The medium should be prepared with the following composition: 60 mM lactobionic acid; 20 mM taurine; 10 mM KH2PO4; 20 mM HEPES; 110 mM D-Sucrose; 0.5 mM EGTA (the concentration needed can be tested previously); 1 g/L BSA (fatty acid free). The pH is adjusted with KOH to 7.1 as for MiR05-Kit, see MiPNet22.10 MiR05-kit for detailed instructions. The MgCl2 should be added only during or right before the experiments.
- For the formulation of the buffer with which the method was described, see: Chinopoulos 2009 ("ANT buffer") and Chinopoulos 2014 Methods Enzymol (buffer A). It is also recommended to prepare this medium without the MgCl2, which should be added only during or right before the experiments.
- For the calibration, MgCl2 is added stepwise in 0.1 mM steps, 10 times (see below, calibration and Kd determination). For experimental runs, 1 to 1.5 mM MgCl2 can be added either directly to the medium aliquot or directly to the O2k chamber before the addition of the sample. Recommendations based on the following publications:
- For isolated mitochondria, 1.0 mM MgCl2 (Chinopoulos 2009)
- For permeabilized cells, 1.5 mM MgCl2 (Chinopoulos 2014 Methods Enzymol).
- For the calibration, MgCl2 is added stepwise in 0.1 mM steps, 10 times (see below, calibration and Kd determination). For experimental runs, 1 to 1.5 mM MgCl2 can be added either directly to the medium aliquot or directly to the O2k chamber before the addition of the sample. Recommendations based on the following publications:
- Note that while Magnesium Green Kd for Mg2+ is 1.0 mM, Magnesium Green Kd for Ca2+ is 6 µM: this means that Magnesium Green binds more strongly to Ca2+ than to Mg2+ and therefore cannot be used in the presence of significant concentrations of free Ca2+. Possible contaminating transition metals should be chelated by a small (µM range) concentration of EGTA, EDTA or DTPA.
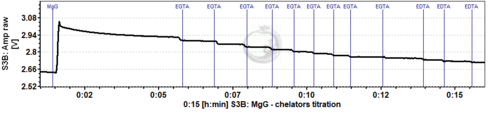
- Titration of EGTA and EDTA
- To test the need to add these chelators, after addition of MgG, substrates and sample to the chamber, titrate EGTA (suggestion: 1 µM steps) until the signal no longer decreases, and then EDTA (suggestion: 1 µM steps) until the signal no longer decreases. This can be performed during the calibration and Kd determination assay. Once the concentrations of EGTA and/or EDTA to be added in the medium are determined, they can be used in the further experiments of mitochondrial ATP production with these substrates and sample. For MiR05 prepared with 500 µM EGTA, it was not necessary to add more chelators (Cardoso 2021 BEC MgG).
- You can determine the necessary concentration of chelators in the first experiments by stepwise titration, and directly add the final concentration of chelator to the medium in subsequent experiments. Please be aware that this should ideally be done for each combination of substrates and each type of sample.
- Example of MgG traces where EGTA and EDTA were titrated (each event shows a titration of 1 µM EGTA or EDTA):
- Titration of EGTA and EDTA
- Inhibitors of ATPases and other enzymes
- Ap5A can be used to inhibit the adenylate kinase, which may consume ADP and produce ATP and AMP (Chinopoulos et al., 2014).
- For samples that present contamination by ATPases and other ATP-consuming enzymes, the use of inhibitors such as sodium orthovanadate and beryllium fluoride (as a mix of sodium fluoride and beryllium sulfate) is recommended (Chinopoulos et al., 2014).
- Pham et al., 2014 used blebbistatin to inhibit myosin heavy chain and ouabain to inhibit Na+,K+-ATPase with rat heart homogenates.
- The medium may be prepared with these inhibitors directly, or the inhibitors may be directly added into the chamber before the experiment starts (before sample addition).
- The use of creatine (e.g. MiR05Cr) is not recommended for this technique since it would activate creatine kinase.
- Inhibitors of ATPases and other enzymes
-
- Colour of media and chemicals used
- For all fluorometric techniques, special care must be taken to avoid colored chemicals that might affect the fluorescence signal (excitation and emission wavelength spectra).
- With MgG fluorophore, some uncouplers that present a yellow color might affect the signal.
- If the uncouplers are added in a SUIT protocol after the ANT inhibitor Carboxyatractyloside, the subsequent steps are not relevant for the MgG assay anymore, only for respiration. Therefore, any uncoupler may be used if the MgG signal will not be analysed.
- To correlate ATP efflux with mt membrane potential, it is recommended to use the uncoupler SF 6847, see: Chinopoulos 2009.
- For all fluorometric techniques, special care must be taken to avoid colored chemicals that might affect the fluorescence signal (excitation and emission wavelength spectra).
- Colour of media and chemicals used
-
Sample
- The MgG technique should always be used with mitochondrial preparations, such as isolated mitochondria, permeabilized cells or tissue homogenates. This method is based on measuring ATP/ADP exchange by ANT. This requires a Mg2+-sensitive fluorescent dye, and a type of sample in which the ADP/ATP molecules are accessible to the dye, meaning that living cells cannot be used for this technique.
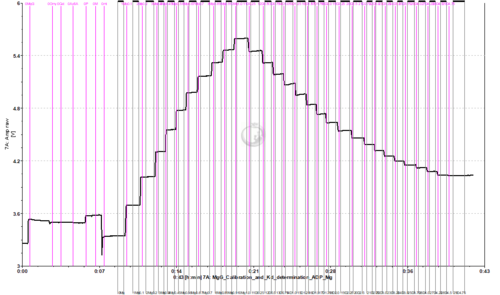
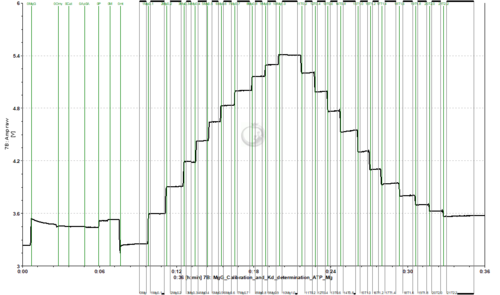
Calibration and Kd determination of ADP or ATP to Mg2+ using MgG
- To assess the exchange of ADP/ATP by ANT using MgG, the Kd of ADP and ATP to Mg2+ should be previously calculated for the pertaining experimental conditions. This can be done in the O2k FluoRespirometer with a protocol in which a series of MgCl2 titrations are performed for calibration up to 1 mM MgCl2, and then a series of titrations with ADP or ATP are performed.
- Add to the chamber the same respiration media that will be used for experiments, without MgCl2.
- Add MgG to the chamber and allow stabilization of the signal if necessary. It is also possible to dilute MgG in the respiration medium prior to addition to the chamber
- Add carboxyatractyloside to inhibit the transport of ADP/ATP, oligomycin to inhibit ATP-synthase activity and Ap5A to inhibit adenylate kinase.
- Add to the chambers the same substrates and sample to be used in the experiments.
- Titrate MgCl2 to the chamber. 10 titrations in 0.1 mM steps are recommended (2 µL of 0.1 M solution).
- Titrate ADP or ATP to the chamber. This must be done separately in two chambers, one for ADP and one for ATP.
- ADP: 0.2 M stock solution, 19 titrations with 2.5 µL (0.25 mM per titration)
- ATP: 0.2 M stock solution, 11 titrations with 2 µL (0.2 mM per titration)
- For performing these protocols, choose the following DLP files: MgG_Calibration_and_Kd_determination_ADP_Mg.DLP and MgG_Calibration_and_Kd_determination_ATP_Mg.DLP in DatLab 7.4.
- Example of traces from calibration with MgCl2 titrations, followed by ADP/ATP titrations for determination of the Kd:
Calibrating the signal and calculating the Kd of ADP or ATP to Mg2+
- For performing the calibration and Kd determination, choose the following Excel template in the folder DL-Protocols\Instrumental of DatLab 7.4: "Template_MgG_Calibration_and_Kd_determination_ADP_and_ATP_to_Mg".
SUIT protocol for mitochondrial ATP production measurement with MgG
- Before starting the SUIT protocol, add MgG and MgCl2 to the chamber: DL-Protocols\Instrumental\MgG calibration\MgG_before_use.DLP
- This DatLab Protocol was prepared for experiments with 1 mM final concentration of MgCl2 (one titration of 2 µL of 1 M stock solution), however, this may be modified if necessary.
- We do not recommend adding the sample to the medium without MgCl2 for respiration/ATP production measurements.
- We do not recommend calibrating the signal in the absence of sample.
- For a SUIT protocol that uses MgG for measurement of mitochondrial ATP production combined with O2 flux measurement, see: SUIT-006 MgG mt D055 or SUIT-006 MgG ce-pce D085, SUIT-006.
- The substrates can be modified to any other combination, however, we do not recommend using more than one combination of substrates/inhibitors in the same protocol for ATP production analysis.
- For protocols with MgG dye, ADP should be prepared without MgCl2, since the technique is based on Mg2+ measurement.
- Before starting the SUIT protocol, add MgG and MgCl2 to the chamber: DL-Protocols\Instrumental\MgG calibration\MgG_before_use.DLP
Can I measure ATP production with MgG in living cells?
- It is not possible to use living cells to measure ATP production with MgG, please find more information under Sample in this page.
Calculating the mitochondrial ATP production
- The calculation of ATP appearing in the medium is done taking into consideration the concentration of ADP added (initial concentration of ADP), the initial ATP concentration, which is considered 0 for no ATP addition, the Mg2+ concentration and the Kd of ADP and ATP for Mg2+. It is recommended to calculate both Kd values for the specific experimental conditions (the medium, substrates and sample used in the experiments).
- For calculating the ATP production, choose the following Excel template in the folder DL-Protocols\SUIT: "Template - MgG ATP production analysis" (a demo file is also provided). The latest version of the template can be downloaded below.
Excel analysis templates
- An Excel analysis template to calculate ATP production is available here.
- The calculations used in the excel analysis template are provided complying with Oroboros transparency policy: [1], [2]
- Last update 2021-09-16: Calibration regression takes 10 points into consideration. This can be changed by the user if needed.
- For SUIT-006 MgG mt D055 protocol, see template: File:SUIT-006 MgG mt D055.xlsx and a demo File:SUIT-006 MgG mt D055 demo.xlsx
- Manual: MiPNet26.10 MgG data analysis
SUITbrowser question: Mitochondrial ATP production
- With the use of MgG, the mitochondrial ATP production can be assessed by high-resolution fluorespirometry.
- Use the SUITbrowser to find the best protocol to answer this and other research questions.
Additional information
Is it possible to measure ATP production by measuring only respiration?
- E.g., in the SUIT-003 O2 ce D009 protocol:
- In this protocol, the ROUTINE respiration of living cells is measured, which is followed by titration of oligomycin, an ATP synthase inhibitor, which will inhibit ATP production. After the oligomycin titration, LEAK respiration will be measured, and this will allow analyzing the free ROUTINE activity, the respiratory activity available for phosphorylation of ADP to ATP (see also: L/R coupling control ratio, NetROUTINE control ratio). However, this is not a direct measurement of ATP production.
- A similar issue, for measurements in mitochondrial preparations in which LEAK- and OXPHOS-respiration can be measured, is discussed in the Bioenergetics Communications Mitochondrial physiology paper: "In general, it is inappropriate to use the term ATP production or ATP turnover for the difference of O2 flux measured in the OXPHOS- and LEAK-states. P-L is the upper limit of OXPHOS-capacity that is freely available for ATP production (corrected for LEAK-respiration) and is fully coupled to phosphorylation with a maximum mechanistic stoichiometry" Gnaiger et al 2020, v1.
Troubleshooting
Publications: MgG
| Year | Reference | Organism | Tissue;cell | Preparations | Stress | Diseases | |
|---|---|---|---|---|---|---|---|
| Arias-Reyes 2023 MitoFit | 2023 | Arias-Reyes C, Aliaga-Raduán F, Pinto-Aparicio R, Joseph V, Soliz J (2023) Mitochondrial plasticity in the retrosplenial cortex enhances ATP synthesis during acclimatization to hypoxia in mice but not in rats. MitoFit Preprints 2023.6. https://doi.org/10.26124/mitofit:2023-0006 | Mouse Rat | Nervous system | Permeabilized tissue | Hypoxia | |
| Quemeneur 2022 Sci Rep | 2022 | Quéméneur JB, Danion M, Cabon J, Collet S, Zambonino-Infante JL, Salin K (2022) The relationships between growth rate and mitochondrial metabolism varies over time. https://doi.org/10.1038/s41598-022-20428-9 | Fishes | Skeletal muscle | Homogenate | ||
| Thoral 2021 Biol Lett | 2021 | Thoral E, Roussel D, Chinopoulos C, Teulier L, Salin K (2021) Low oxygen levels can help to prevent the detrimental effect of acute warming on mitochondrial efficiency in fish. Biol Lett 17:20200759. | Fishes | Skeletal muscle | Homogenate | ||
| Willis 2021 Sci Rep | 2021 | Willis JR, Hickey AJR, Devaux JBL (2021) Thermally tolerant intertidal triplefin fish (Tripterygiidae) sustain ATP dynamics better than subtidal species under acute heat stress. Sci Rep 11:11074. | Fishes | Nervous system | Homogenate | ||
| Salin 2021 Mar Environ Res | 2021 | Salin K, Mathieu-Resuge M, Graziano N, Dubillot E, Le Grand F, Soudant P, Vagner M (2021) The relationship between membrane fatty acid content and mitochondrial efficiency differs within- and between- omega-3 dietary treatments. Mar Environ Res 163:105205. | Fishes | Skeletal muscle | Isolated mitochondria | ||
| Cardoso 2021 BEC MgG | 2021 | Cardoso LHD, Doerrier C, Gnaiger E (2021) Magnesium Green for fluorometric measurement of ATP production does not interfere with mitochondrial respiration. Bioenerg Commun 2021.1. https://doi.org/10.26124/bec:2021-0001 | Mouse | Heart | Isolated mitochondria | ||
| Devaux 2019 Front Physiol | 2019 | Devaux JBL, Hedges CP, Birch N, Herbert N, Renshaw GMC, Hickey AJR (2019) Acidosis maintains the function of brain mitochondria in hypoxia-tolerant triplefin fish: a strategy to survive acute hypoxic exposure? Front Physiol 9:1941. | Fishes | Nervous system | Permeabilized tissue Isolated mitochondria | Oxidative stress;RONS Hypoxia | |
| Salin 2019 Proc Biol Sci | 2019 | Salin K, Villasevil EM, Anderson GJ, Lamarre SG, Melanson CA, McCarthy I, Selman C, Metcalfe NB (2019) Differences in mitochondrial efficiency explain individual variation in growth performance. Proc Biol Sci 286:20191466. | Fishes | Skeletal muscle Liver | Homogenate | ||
| Salin 2018 Integr Comp Biol | 2018 | Salin K, Villasevil EM, Anderson GJ, Selman C, Chinopoulos C, Metcalfe NB (2018) The RCR and ATP/O indices can give contradictory messages about mitochondrial efficiency. Integr Comp Biol 58:486-94. | Fishes | Liver | Homogenate | ||
| Masson 2017 Sci Rep | 2017 | Masson SWC, Hedges CP, Devaux JBL, James CS, Hickey AJR (2017) Mitochondrial glycerol 3-phosphate facilitates bumblebee pre-flight thermogenesis. Sci Rep 7:13107. | Hexapods | Skeletal muscle | Permeabilized tissue | ||
| Napa 2017 Int J Dent | 2017 | Napa K, Baeder AC, Witt JE, Rayburn ST, Miller MG, Dallon BW, Gibbs JL, Wilcox SH, Winden DR, Smith JH, Reynolds PR, Bikman BT (2017) LPS from P. gingivalis negatively alters gingival cell mitochondrial bioenergetics. Int J Dent 2017:2697210. | Human | Endothelial;epithelial;mesothelial cell | Permeabilized cells | ||
| Power 2016 Am J Physiol Heart Circ Physiol | 2016 | Power AS, Pham T, Loiselle DS, Crossman DH, Ward ML, Hickey AJ (2016) Impaired ADP channeling to mitochondria and elevated reactive oxygen species in hypertensive hearts. https://doi.org/10.1152/ajpheart.00050.2016 | Rat | Heart | Permeabilized tissue Homogenate | Cardiovascular | |
| Salin 2016 Physiol Rep | 2016 | Salin K, Villasevil EM, Auer SK, Anderson GJ, Selman C, Metcalfe NB, Chinopoulos C (2016) Simultaneous measurement of mitochondrial respiration and ATP production in tissue homogenates and calculation of effective P/O ratios. Physiol Rep 10.14814/phy2.13007. | Fishes | Liver | Homogenate | ||
| Power 2014 Physiol Rep | 2014 | Power A, Pearson N, Pham T, Cheung C, Phillips A, Hickey A (2014) Uncoupling of oxidative phosphorylation and ATP synthase reversal within the hyperthermic heart. Physiol Rep pii:e12138. | Rat | Heart | Isolated mitochondria | Temperature | |
| Chinopoulos 2014 Methods Enzymol | 2014 | Chinopoulos C, Kiss G, Kawamata H, Starkov AA (2014) Measurement of ADP-ATP exchange in relation to mitochondrial transmembrane potential and oxygen consumption. Methods Enzymol 542:333-48. doi:10.1016/B978-0-12-416618-9.00017-0 | Human | HEK | Permeabilized cells | ||
| Pham 2014 Am J Physiol | 2014 | Pham T, Loiselle D, Power A, Hickey AJ (2014) Mitochondrial inefficiencies and anoxic ATP hydrolysis capacities in diabetic rat heart. Am J Physiol 307:C499–507. | Rat | Heart | Homogenate | Ischemia-reperfusion Oxidative stress;RONS Mitochondrial disease | Diabetes Myopathy |
| Goo 2013 Clin Exp Pharmacol Physiol | 2013 | Goo S, Pham T, Han JC, Nielsen P, Taberner A, Hickey A, Loiselle D (2013) Multiscale measurement of cardiac energetics. Clin Exp Pharmacol Physiol 40:671-81. | Rat | Heart | Permeabilized cells Homogenate Isolated mitochondria | Oxidative stress;RONS | |
| Iftikar 2013 PLoS One | 2013 | Iftikar FI, Hickey AJ (2013) Do mitochondria limit hot fish hearts? Understanding the role of mitochondrial function with heat stress in Notolabrus celidotus. PLoS One 8:e64120. | Fishes | Heart | Permeabilized tissue | Oxidative stress;RONS | |
| Chinopoulos 2009 | 2009 | Chinopoulos C, Vajda S, Csanady L, Mandi M, Mathe K, Adam-Vizi V (2009) A Novel Kinetic Assay of Mitochondrial ATP-ADP Exchange Rate Mediated by the ANT. Biophys J 96, 2490-504. doi:10.1016/j.bpj.2008.12.3915 | Rat | Heart Nervous system Liver | Isolated mitochondria |
- Abstracts: MgG