Missaglia 2021 Crit Rev Biochem Mol Biol
| Missaglia S, Tavian D, Angelini C (2021) ETF dehydrogenase advances in molecular genetics and impact on treatment. Crit Rev Biochem Mol Biol 56:360-72. doi: 10.1080/10409238.2021.1908952 |
Missaglia S, Tavian D, Angelini C (2021) Crit Rev Biochem Mol Biol
Abstract: Electron transfer flavoprotein dehydrogenase, also called ETF-ubiquinone oxidoreductase (ETF-QO), is a protein localized in the inner membrane of mitochondria, playing a central role in the electron-transfer system. Indeed, ETF-QO mediates electron transport from flavoprotein dehydrogenases to the ubiquinone pool. ETF-QO mutations are often associated with riboflavin-responsive multiple acyl-CoA dehydrogenase deficiency (RR-MADD, OMIM#231680), a multisystem genetic disease characterized by various clinical manifestations with different degrees of severity. In this review, we outline the clinical features correlated with ETF-QO deficiency and the benefits obtained from different treatments, such as riboflavin, L-carnitine and/or coenzyme Q10 supplementation, and a diet poor in fat and protein. Moreover, we provide a detailed summary of molecular and bioinformatic investigations, describing the mutations identified in ETFDH gene and highlighting their predicted impact on enzymatic structure and activity. In addition, we report biochemical and functional analysis, performed in HEK293 cells and patient fibroblasts and muscle cells, to show the relationship between the nature of ETFDH mutations, the variable impairment of enzyme function, and the different degrees of RR-MADD severity. Finally, we describe in detail 5 RR-MADD patients carrying different ETFDH mutations and presenting variable degrees of clinical symptom severity.
• Bioblast editor: Gnaiger E
Labels:
Pathway: F
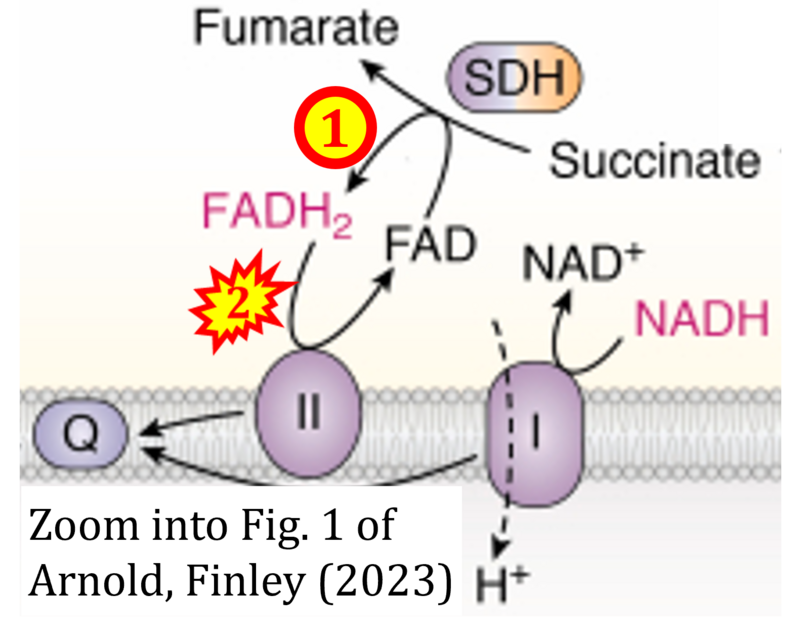
Correction: FADH2 and S-pathway
- A commonly found error on FADH2 in the S-pathway requires correction. For clarification, see page 48 in Gnaiger (2020)
- Quote (p 48): "The substrate of CII is succinate, which is oxidized forming fumarate while reducing flavin adenine dinucleotide FAD to FADH2, with further electron transfer to the quinone pool. Whereas reduced NADH is a substrate of Complex I linked to dehydrogenases of the TCA cycle and mt-matrix upstream of CI, reduced FADH2 is a product of Complex II with downstream electron flow from CII to Q."
- A commonly found error on FADH2 in the S-pathway requires correction. For clarification, see page 48 in Gnaiger (2020)
- Gnaiger E (2020) Mitochondrial pathways and respiratory control. An introduction to OXPHOS analysis. 5th ed. Bioenerg Commun 2020.2. https://doi.org/10.26124/bec:2020-0002