Difference between revisions of "Succinate control state"
From Bioblast
| Line 4: | Line 4: | ||
|info=[[Gnaiger 2014 MitoPathways |Gnaiger 2014 MitoPathways - Chapter 4.2]] | |info=[[Gnaiger 2014 MitoPathways |Gnaiger 2014 MitoPathways - Chapter 4.2]] | ||
}} | }} | ||
{{MitoPedia concepts}} | {{MitoPedia concepts | ||
|mitopedia concept=Respiratory state, SUIT state | |||
}} | |||
{{MitoPedia methods}} | {{MitoPedia methods}} | ||
{{MitoPedia O2k and high-resolution respirometry | {{MitoPedia O2k and high-resolution respirometry}} | ||
}} | |||
{{MitoPedia topics}} | {{MitoPedia topics}} | ||
[[File:S.jpg|right|400px|link=Gnaiger 2014 MitoPathways |Gnaiger 2014 MitoPathways - Chapter 4.2]] | [[File:S.jpg|right|400px|link=Gnaiger 2014 MitoPathways |Gnaiger 2014 MitoPathways - Chapter 4.2]] | ||
Revision as of 16:37, 13 May 2016
Description
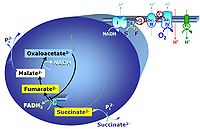
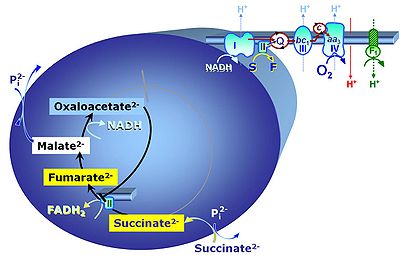
S: When succinate is added without rotenone, oxaloacetate is formed from malate by the action of malate dehydrogenase. Oxaloacetate accumulates and is a more potent competitive inhibitor of succinate dehydrogenase than malonate even at small concentration. Reverse electron flow from CII to CI is known to stimulate production of reactive oxygen species under these conditions to extremely high, nonphysiological levels. Addition of malate reduces superoxide production with succinate, probably due to a shift in the redox state and oxaloacetate inhibition of CII. Compare: Complex II-linked substrate state.
Abbreviation: S
Reference: Gnaiger 2014 MitoPathways - Chapter 4.2
MitoPedia concepts:
Respiratory state,
SUIT state
S(L)
S(P)
S(E)
Details
- OXPHOS capacity with succinate alone, SD, is 30-40% lower than flux with succinate and rotenone, S(Rot)D, in human and rat skeletal muscle mitochondria, due to inhibition of succinate dehydrogenase by accumulating oxaloacetate. This is similar in mammalian liver, where >10% of malic enzyme is cytosolic.
- Note: Erroneously '<10%' has been typed in the original text (Gnaiger 2014 MitoPathways).
- Conditions with succinate as the sole substrate are different if mt-malic enzyme plays a significant role, as is the case in proliferating cells. Measurement of the ~P/O2 ratio in CII-linked respiration with and without rotenone provides an indication of the extent to which CI is stimulated by NADH, which is generated through the mtME and PDH pathway in parallel with formation and removal of oxaloacetate through the MDH-CS pathway. The exported malate may be too dilute for an active antiport against citrate and 2-oxoglutarate (compare malate with mtME). However, generation of NADH in the presence of mtME activity will counteract reverse CII to CI electron transfer and thus play an important role in the regulation of ROS production under these experimental conditions.