Difference between revisions of "Template:Oxygen sensor test"
From Bioblast
Garcia Luiz (talk | contribs) (Undo revision 204357 by Garcia Luiz (talk)) Tag: Undo |
|||
| (10 intermediate revisions by 5 users not shown) | |||
| Line 1: | Line 1: | ||
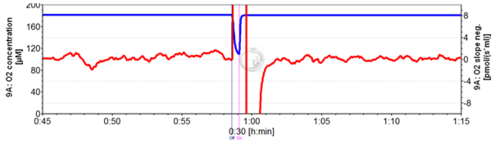
::: ''' | [[File:Stirrer test.png|thumb|right|500px|'''Stirrer test''' for quality control (standard 30 s) with 30 min time scale displayed with Graph Layout “02-Calibration - Background” (MiR05; 37 °C; data recording interval: 2 s; slope smoothing: 40 data points).]] | ||
::: '''Oxygen sensor test''' | |||
[[ | :::: The oxygen sensor test starts in an [[open chamber]]. | ||
:::<span style="background-color:#00FF00; border-color:#D4D0D0; border-style: solid; border-width: 1px; margin: 2px">'''1.'''</span> Before final equilibration, perform a [[Stirrer_test|stirrer test]] [F9], switching both stirrers automatically off for 30 s. | |||
::: | :::<span style="background-color:#00FF00; border-color:#D4D0D0; border-style: solid; border-width: 1px; margin: 2px">'''2.'''</span> About 20 minutes are required for air equilibration after temperature equilibration of the incubation medium, visualized as stabilization of the Peltier power (Fig. Quality control; time scale is 01:10 hh:mm). | ||
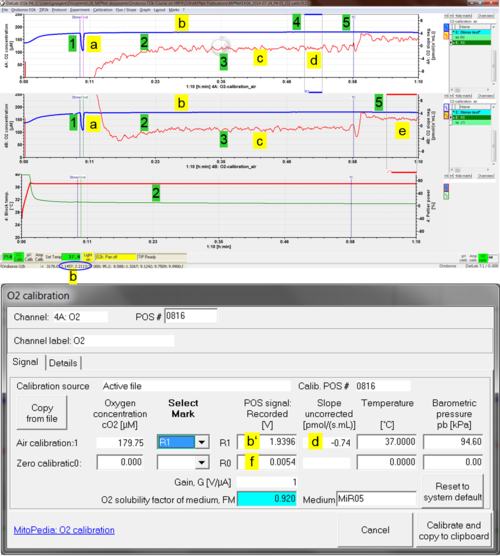
: | ::::* Quality control label <span style="background-color:#FFFF00; border-color:#D4D0D0; border-style: solid; border-width: 1px; margin: 2px">'''a'''</span>: Upon automatic re-start of the stirrer (On), the increase of the oxygen signal should be rapid and monoexponential. | ||
::: | :::::* Quality control label <span style="background-color:#FFFF00; border-color:#D4D0D0; border-style: solid; border-width: 1px; margin: 2px">'''b'''</span>: The raw signal (blue plot; 1 V = 1 µA at Gain 1) should be 1 to 3 V at 25 to 37 °C at sea level up to 1000 m (''p''<sub>b</sub> 101 to 90 kPa). At a Gain setting of 2 the raw signal [V] is multiplied by 2. | ||
[[File:O2 calibration air.png|thumb|right|500px|'''Quality control''']] | |||
::: | :::<span style="background-color:#00FF00; border-color:#D4D0D0; border-style: solid; border-width: 1px; margin: 2px">'''3.'''</span> Within 40 minutes, the oxygen signals should be stable with an O2 slope (uncorrected) close to zero. | ||
:::# Set a mark on the oxygen signal (R1) and click on <span style="background:yellow">O2 Calib.</span style> to open the DatLab O2 calibration window. | ::::* Quality control label <span style="background-color:#FFFF00; border-color:#D4D0D0; border-style: solid; border-width: 1px; margin: 2px">'''c'''</span>: Signal noise should be low, reflected in a noise of the O2 slope (red plot) within ± 2 (± 4 is acceptable) pmol∙s<sup>−1</sup>∙mL<sup>−1</sup> at a data recording interval of 2 s and 40 data points selected for calculation of the slope. | ||
::: | :::<span style="background-color:#00FF00; border-color:#D4D0D0; border-style: solid; border-width: 1px; margin: 2px">'''4.'''</span> Set a mark on the oxygen signal (R1) and click on <span style="background:yellow">O2 Calib.</span style> to open the DatLab O2 calibration window ([[MiPNet06.03 POS-calibration-SOP]]). | ||
:::# | ::::* Quality control label <span style="background-color:#FFFF00; border-color:#D4D0D0; border-style: solid; border-width: 1px; margin: 2px">'''d'''</span>: The slope uncorrected should be within ± 1 pmol∙s<sup>−1</sup>∙mL<sup>−1</sup> averaged across the section of the experiment marked as R1 for air calibration (d). The recorded POS signal should be close to the previous calibration under identical experimental conditions. See O2-Calibration window (see Fig. right, label <span style="background-color:#FFFF00; border-color:#D4D0D0; border-style: solid; border-width: 1px; margin: 2px">'''b’'''</span>). | ||
::: | :::<span style="background-color:#00FF00; border-color:#D4D0D0; border-style: solid; border-width: 1px; margin: 2px">'''5.'''</span> Close the chamber and if required, perform a zero oxygen calibration. | ||
::: | ::::* Quality control label <span style="background-color:#FFFF00; border-color:#D4D0D0; border-style: solid; border-width: 1px; margin: 2px">'''e'''</span>: After closing the chamber, select plot Y2 and set mark J°1. O<sub>2</sub> slope neg. should be within 3.0 ± 1 pmol∙s<sup>−1</sup>∙mL<sup>−1</sup>. | ||
::: | :::::: O<sub>2</sub> slope neg. values higher than 4.0 pmol∙s<sup>−1</sup>∙mL<sup>−1</sup> indicate: | ||
::: | :::::::» [[Biological contamination]]. | ||
::: | :::::::» Air bubbles in the [[Closed_chamber | Closed chamber]]: switch on the illumination of the O2k and inspect the O2k-Chamber through the front window. Remove any air bubbles. | ||
::: | :::::::» A large volume of medium collected in the receptacle of the stopper: siphon off excess medium. | ||
::: | :::::::» A larger chamber volume: check [[O2k-Chamber volume calibration]]. | ||
::::* Quality control label <span style="background-color:#FFFF00; border-color:#D4D0D0; border-style: solid; border-width: 1px; margin: 2px">'''f'''</span>: The zero signal at mark R0 for zero calibration should be <2 % of R1 (stable at <5 % is acceptable). | |||
Latest revision as of 14:18, 25 February 2022
- Oxygen sensor test
- The oxygen sensor test starts in an open chamber.
- 1. Before final equilibration, perform a stirrer test [F9], switching both stirrers automatically off for 30 s.
- 2. About 20 minutes are required for air equilibration after temperature equilibration of the incubation medium, visualized as stabilization of the Peltier power (Fig. Quality control; time scale is 01:10 hh:mm).
- Quality control label a: Upon automatic re-start of the stirrer (On), the increase of the oxygen signal should be rapid and monoexponential.
- Quality control label b: The raw signal (blue plot; 1 V = 1 µA at Gain 1) should be 1 to 3 V at 25 to 37 °C at sea level up to 1000 m (pb 101 to 90 kPa). At a Gain setting of 2 the raw signal [V] is multiplied by 2.
- 3. Within 40 minutes, the oxygen signals should be stable with an O2 slope (uncorrected) close to zero.
- Quality control label c: Signal noise should be low, reflected in a noise of the O2 slope (red plot) within ± 2 (± 4 is acceptable) pmol∙s−1∙mL−1 at a data recording interval of 2 s and 40 data points selected for calculation of the slope.
- 4. Set a mark on the oxygen signal (R1) and click on O2 Calib. to open the DatLab O2 calibration window (MiPNet06.03 POS-calibration-SOP).
- Quality control label d: The slope uncorrected should be within ± 1 pmol∙s−1∙mL−1 averaged across the section of the experiment marked as R1 for air calibration (d). The recorded POS signal should be close to the previous calibration under identical experimental conditions. See O2-Calibration window (see Fig. right, label b’).
- 5. Close the chamber and if required, perform a zero oxygen calibration.
- Quality control label e: After closing the chamber, select plot Y2 and set mark J°1. O2 slope neg. should be within 3.0 ± 1 pmol∙s−1∙mL−1.
- O2 slope neg. values higher than 4.0 pmol∙s−1∙mL−1 indicate:
- » Biological contamination.
- » Air bubbles in the Closed chamber: switch on the illumination of the O2k and inspect the O2k-Chamber through the front window. Remove any air bubbles.
- » A large volume of medium collected in the receptacle of the stopper: siphon off excess medium.
- » A larger chamber volume: check O2k-Chamber volume calibration.
- O2 slope neg. values higher than 4.0 pmol∙s−1∙mL−1 indicate:
- Quality control label f: The zero signal at mark R0 for zero calibration should be <2 % of R1 (stable at <5 % is acceptable).
- 3. Within 40 minutes, the oxygen signals should be stable with an O2 slope (uncorrected) close to zero.