Difference between revisions of "Gibbs energy"
From Bioblast
| Line 16: | Line 16: | ||
<big>'''Eq. 2''': d<sub>tr</sub>''G'' = ''µ''<sub>A</sub>·d<sub>tr</sub>''n''<sub>A</sub> + ''µ''<sub>B</sub>·d<sub>tr</sub>''n''<sub>B</sub> [J] </big> | <big>'''Eq. 2''': d<sub>tr</sub>''G'' = ''µ''<sub>A</sub>·d<sub>tr</sub>''n''<sub>A</sub> + ''µ''<sub>B</sub>·d<sub>tr</sub>''n''<sub>B</sub> [J] </big> | ||
:::: where the advancement d<sub>tr</sub>''ξ''<sub>''i''</sub> (''i'' = A or B) is | :::: where the [[advancement]] d<sub>tr</sub>''ξ''<sub>''i''</sub> (''i'' = A or B) is | ||
<big>'''Eq. 3''': d<sub>tr</sub>''ξ''<sub>''i''</sub> = d<sub>tr</sub>''n''<sub>A</sub>·''ν''<sub>A</sub><sup>-1</sup> = d<sub>tr</sub>''n''<sub>B</sub>·''ν''<sub>B</sub><sup>-1</sup> [mol] </big> | <big>'''Eq. 3''': d<sub>tr</sub>''ξ''<sub>''i''</sub> = d<sub>tr</sub>''n''<sub>A</sub>·''ν''<sub>A</sub><sup>-1</sup> = d<sub>tr</sub>''n''<sub>B</sub>·''ν''<sub>B</sub><sup>-1</sup> [mol] </big> | ||
Revision as of 14:04, 18 October 2022
Description
Gibbs energy G [J] is exergy which cannot be created internally (subscript i), but in contrast to internal-energy (diU/dt = 0) is not conserved but is dissipated (diG/dt < 0) in irreversible energy transformations at constant temperature and (barometric) pressure, T,p. Exergy is available as work in reversible energy transformations, and can be partially conserved when the exergonic transformation is coupled to an endergonic transformation.
Abbreviation: G [J]
Reference: Energy
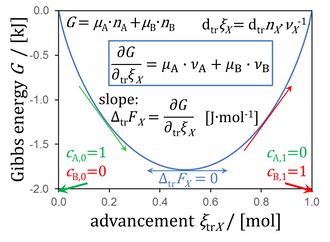
Figure 8.5. Gibbs energy as a function of advancement of a transformation (0 = -1 A + 1 B) in a closed isothermal system at constant pressure, for μA° = μB° = 0 kJ·mol-1 (modified from Gnaiger 2020 BEC MitoPathways).
Gibbs energy as a function of advancement
Communicated by Gnaiger E 2022-10-18
- In a transformation tr 0 = -1 A +1 B proceeding in a system with volume V at constant barometric pressure p, the Gibbs energy of reactants A and B is
Eq. 1: G = µA·nA + µB·nB [J]
- A small change dG at constant chemical potentials µi is due to a small advancement of the transformation, in closed or open isothermal systems, exchanging heat in equilibrium with an external thermostat at constant temperature,
Eq. 2: dtrG = µA·dtrnA + µB·dtrnB [J]
- where the advancement dtrξi (i = A or B) is
Eq. 3: dtrξi = dtrnA·νA-1 = dtrnB·νB-1 [mol]
- The isomorphic force of transformation ΔtrFX is the derivative of exergy per advancement,
Eq. 4: ΔtrFX = ∂G/∂trξX [J·mol-1]
- Note that ∂G ≝ dtrG. Then inserting Eq. 2 and Eq. 3 into Eq. 4, the force of transformation is expressed as
Eq. 5: ΔtrFX = (µA·dtrnA + µB·dtrnB)/(dtrnB·νB-1) [J·mol-1]
- which can be rewritten as
Eq. 6: ΔtrFX = µA·dtrnA/(dtrnA·νA-1) + µB·dtrnB/(dtrnB·νB-1) [J·mol-1]
- This yields the force as the sum of stoichiometric potentials, summarized in Figure 8.5 (Chapter 8; Gnaiger 2020 BEC MitoPathways),
Eq. 7: ΔtrFX = µA·νA + µB·νB [J·mol-1]
- In general,
Eq. 8: ΔtrFX = Σµi·νi = ΣFtri[J·mol-1]
- It may arouse curiosity, why the sign of difference Δ is used in the symbol, whereas the equation suggest a sum Σ in contrast to a difference. This is best explained by the fact that in various conventional contexts — such as the classical formulation of the pmF — the stoichiometric numbers (-1 and +1) are omitted, which yields the difference,
Eq. 9: ΔtrFX ≡ µB - µA [J·mol-1]
- The conceptual importance of the stoichiometric numbers — as in dtrnA·νA-1 (Eq. 3) — is emphasized by defining the term stoichiometric potential (Gnaiger 2020; see Eqs. 7 and 8),
Eq. 10: Ftri = µi·νi [J·mol-1]
References
- Gnaiger E (2020) Mitochondrial pathways and respiratory control. An introduction to OXPHOS analysis. 5th ed. Bioenerg Commun 2020.2. https://doi.org/10.26124/bec:2020-0002
- »Bioblast links: Energy and exergy - >>>>>>> - Click on [Expand] or [Collapse] - >>>>>>>
- Units
- Joule [J]; 1 J = 1 N·m = 1 V·C; 1 cal = 4.184 J
- Units
- Fundamental relationships
- » Energy
- » Exergy
- » Extensive quantity
- Fundamental relationships
- Contrast
- » Force
- » Pressure
- » Intensive quantity
- Contrast
- Forms of energy
- » Internal-energy dU
- » Enthalpy dH
- » Heat deQ
- » Bound energy dB
- Forms of energy
- Forms of exergy
- » Helmholtz energy dA
- » Gibbs energy dG
- » Work deW
- » Dissipated energy diD
- Forms of exergy
MitoPedia concepts:
Ergodynamics
