Difference between revisions of "Succinate"
| (33 intermediate revisions by 10 users not shown) | |||
| Line 1: | Line 1: | ||
{{MitoPedia | {{MitoPedia | ||
|abbr=S | |abbr=S | ||
|description=''' | |description=[[File:Succinic_acid.jpg|left|100px|Succinic acid]] | ||
|info=[ | '''Succinic acid''', C<sub>4</sub>H<sub>6</sub>O<sub>4</sub>, (butanedioic acid) is a dicarboxylic acid which occurs under physiological conditions as the anion '''succinate<sup>2-</sup>, S''', with ''p''K<sub>a1</sub> = 4.2 and ''p''K<sub>a2</sub> = 5.6. Succinate is formed in the [[TCA cycle]], and is a substrate of [[Complex II |CII]], reacting to [[fumarate]] and feeding electrons into the [[Q-junction]]. Succinate (CII-linked) and NADH (CI-linked) provide convergent electron entries into the Q-junction. Succinate is transported across the inner mt-membrane by the [[dicarboxylate carrier]]. The plasma membrane of many cell types is impermeable for succinate (but see [[Zhunussova 2015 Am J Cancer Res]] for an exception). Incubation of mt-preparations by succinate alone may lead to accumulation of [[oxaloacetate]], which is a potent inhibitor of Complex II (compare [[Succinate and rotenone]]). High activities of mt-[[Malic enzyme]] (mtME) prevent accumulation of oxaloacetate in incubations with succinate without rotenone. | ||
|info=[[Gnaiger 2020 BEC MitoPathways]], [[Tretter 2016 Biochim Biophys Acta]] | |||
}} | }} | ||
{{ | __TOC__ | ||
[[File:S.jpg|400px|thumb|Succinate, S. From [[Gnaiger 2020 BEC MitoPathways]].]] | |||
| | == Application in [[HRR]] == | ||
| | {{Chemical_description | ||
}} | |abbr=S | ||
|trivial name=Succinate | |||
|complete name=Sodium succinate dibasic hexahydrate | |||
|chem formula= C<sub>4</sub>H<sub>4</sub>0<sub>4</sub>Na<sub>2</sub> * 6H<sub>2</sub>O | |||
|molar mass=270.1 | |||
|vendor=Sigma-Aldrich | |||
|product number=S2378 | |||
|store at=RT | |||
|sensitivity= | |||
|cas=6106-21-4 | |||
|h statements= | |||
|h info= | |||
}}<!--:::'''S: Succinate''' (Succinate disodium salt, hexahydrate, C<sub>4</sub>H<sub>4</sub>0<sub>4</sub>Na<sub>2</sub> * (H<sub>2</sub>O)<sub>6</sub>; Sigma S 2378, 100 g, store at RT; M = 270.1 g·mol<sup>-1</sup>)--> | |||
:::: In the absence of CI-linked substrates, add the CI-inhibitor [[rotenone]] before addition of succinate, to avoid accumulation of [[oxaloacetate]] with subsequent inhibition of [[succinate dehydrogenase]]. See: [[Succinate and rotenone]]. | |||
:::: When keeping the succinate stock solution on ice, check for complete solubilization of succinate and warm the stock solution in your hands if necessary. | |||
:::: '''Preparation of 1 M stock solution:''' | |||
::::# Weight 1.3505 g of succinate and dissolve in 3 mL H<sub>2</sub>O; | |||
::::# Check pH and adjust to 7.0 if necessary with 1 M HCl (about 65 µL will be needed); | |||
::::# Transfer to 5 mL volumetric glass flask and adjust the final volume to 5 mL; | |||
::::# Divide into 0.5 mL portions; | |||
::::# Store frozen at -20 °C. | |||
''' | :::» '''O2k manual titrations''' [[MiPNet09.12 O2k-Titrations]] | ||
''' | ::::* Titration volume ('''2-mL O2k-chamber'''): 20 µL using a 50 µL Hamilton syringe. | ||
::::* Titration volume ('''0.5-mL O2k-chamber'''): 5 µL using a 25 µL Hamilton syringe | |||
::::* Final concentration: 10 mM. Note: The concentration of succinate may be increased up to 50 mM after [[rotenone]] to compensate for the inhibitory effect of [[malate]]. | |||
== [[SUITbrowser]] question: Succinate pathway == | |||
:::: The [https://suitbrowser.oroboros.at/ SUITbrowser] can be used to find the best SUIT protocols to analyze the succinate pathway, among other research questions. | |||
{{MitoPedia topics | |||
|mitopedia topic=Substrate and metabolite | |||
}} | |||
Latest revision as of 15:06, 17 December 2022
Description
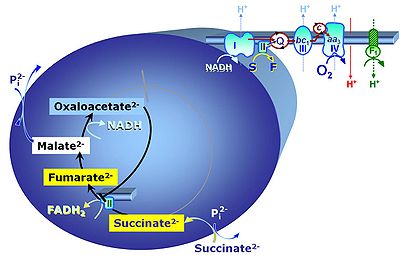
Succinic acid, C4H6O4, (butanedioic acid) is a dicarboxylic acid which occurs under physiological conditions as the anion succinate2-, S, with pKa1 = 4.2 and pKa2 = 5.6. Succinate is formed in the TCA cycle, and is a substrate of CII, reacting to fumarate and feeding electrons into the Q-junction. Succinate (CII-linked) and NADH (CI-linked) provide convergent electron entries into the Q-junction. Succinate is transported across the inner mt-membrane by the dicarboxylate carrier. The plasma membrane of many cell types is impermeable for succinate (but see Zhunussova 2015 Am J Cancer Res for an exception). Incubation of mt-preparations by succinate alone may lead to accumulation of oxaloacetate, which is a potent inhibitor of Complex II (compare Succinate and rotenone). High activities of mt-Malic enzyme (mtME) prevent accumulation of oxaloacetate in incubations with succinate without rotenone.
Abbreviation: S
Reference: Gnaiger 2020 BEC MitoPathways, Tretter 2016 Biochim Biophys Acta
Application in HRR
- S: Succinate (Sodium succinate dibasic hexahydrate; C4H404Na2 * 6H2O), Sigma-Aldrich: S2378, store at RT, CAS: 6106-21-4, M = 270.1 g·mol-1
- In the absence of CI-linked substrates, add the CI-inhibitor rotenone before addition of succinate, to avoid accumulation of oxaloacetate with subsequent inhibition of succinate dehydrogenase. See: Succinate and rotenone.
- When keeping the succinate stock solution on ice, check for complete solubilization of succinate and warm the stock solution in your hands if necessary.
- Preparation of 1 M stock solution:
- Weight 1.3505 g of succinate and dissolve in 3 mL H2O;
- Check pH and adjust to 7.0 if necessary with 1 M HCl (about 65 µL will be needed);
- Transfer to 5 mL volumetric glass flask and adjust the final volume to 5 mL;
- Divide into 0.5 mL portions;
- Store frozen at -20 °C.
- » O2k manual titrations MiPNet09.12 O2k-Titrations
- Titration volume (2-mL O2k-chamber): 20 µL using a 50 µL Hamilton syringe.
- Titration volume (0.5-mL O2k-chamber): 5 µL using a 25 µL Hamilton syringe
- Final concentration: 10 mM. Note: The concentration of succinate may be increased up to 50 mM after rotenone to compensate for the inhibitory effect of malate.
SUITbrowser question: Succinate pathway
- The SUITbrowser can be used to find the best SUIT protocols to analyze the succinate pathway, among other research questions.
MitoPedia topics:
Substrate and metabolite