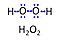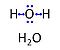- high-resolution terminology - matching measurements at high-resolution
MitoPedia: Substrates and metabolites
The MitoPedia terminology is developed continuously in the spirit of Gentle Science.
| Term | Abbreviation | Description |
|---|---|---|
| 2-Hydroxyglutarate | 2HG | Reduction of oxoglutarate (2OG or alpha-ketoglutarate) to 2-hydroxyglutarate (2HG) is driven by NADPH. 2HG is also formed in side reactions of lactate dehydrogenase and malate dehydrogenase. Millimolar 2HG concentrations are found in some cancer cells compared to , whereas side activities of lactate and malate dehydrogenase form submillimolar s-2-hydroxyglutarate (s-2HG). However, even wild-type IDH1 and IDH2, notably under shifts toward reductive carboxylation glutaminolysis or changes in other enzymes, lead to “intermediate” 0.01–0.1 mM 2HG levels, for example, in breast carcinoma compared with nanomolar concentrations in benign cells. 2HG is considered an important player in reprogramming metabolism of cancer cells. |
| ATP | T | Adenosine triphosphate is a nucleotid and functions as the major carrier of chemical energy in the cells. As it transfers its energy to other molecules, it looses its terminal phosphate group and becomes adenosine diphosphate (ADP). |
| Acetyl-CoA | Acetyl-CoA, C23H38N7O17P3S, is a central piece in metabolism involved in several biological processes, but its main role is to deliver the acetyl group into the TCA cycle for its oxidation. It can be synthesized in different pathways: (i) in glycolysis from pyruvate, by pyruvate dehydrogenase, which also forms NADH; (ii) from fatty acids β-oxidation, which releases one acetyl-CoA each round; (iii) in the catabolism of some amino acids such as leucine, lysine, phenylalanine, tyrosine and tryptophan.
In the mitochondrial matrix, acetyl-CoA is condensed with oxaloacetate to form citrate through the action of citrate synthase in the tricarboxylic acid cycle. Acetyl-CoA cannot cross the mitochondrial inner membrane but citrate can be transported out of the mitochondria. In the cytosol, citrate can be converted to acetyl-CoA and be used in the synthesis of fatty acid, cholesterol, ketone bodies, acetylcholine, and other processes. | |
| Activity | a | The activity (relative activity) is a dimensionless quantity related to the concentration or partial pressure of a dissolved substance. The activity of a dissolved substance B equals the concentration, cB [mol·L-1], at high dilution divided by the unit concentration, c° = 1 mol·L-1:
aB = cB/c° This simple relationship applies frequently to substances at high dilutions <10 mmol·L-1 (<10 mol·m-3). In general, the concentration of a solute has to be corrected for the activity coefficient (concentration basis), γB, aB = γB·cB/c° At high dilution, γB = 1. In general, the relative activity is defined by the chemical potential, µB aB = exp[(µB-µ°)/RT] |
| Acylcarnitine | AC | Acylcarnitines are esters derivative of carnitine and fatty acids, involved in the metabolism of fatty acids. Long-chain acylcarnitines such as palmitoylcarnitine must be transported in this form, conjugated to carnitine, into the mitochondria to deliver fatty acids for fatty acid oxidation and energy production. Medium-chain acylcarnitines such as octanoylcarnitine are also frequently used for high-resolution respirometry. |
| Additive effect of convergent electron flow | Aα&β | Additivity Aα&β describes the principle of substrate control of mitochondrial respiration with convergent electron flow. The additive effect of convergent electron flow is a consequence of electron flow converging at the Q-junction from respiratory Complexes I and II (NS or CI&II e-input). Further additivity may be observed by convergent electron flow through glycerophosphate dehydrogenase and electron-transferring flavoprotein Complex. Convergent electron flow corresponds to the operation of the TCA cycle and mitochondrial substrate supply in vivo. Physiological substrate combinations supporting convergent NS e-input are required for reconstitution of intracellular TCA cycle function. Convergent electron flow simultaneously through Complexes I and II into the Q-junction supports higher OXPHOS capacity and ET capacity than separate electron flow through either CI or CII. The convergent NS effect may be completely or partially additive, suggesting that conventional bioenergetic protocols with mt-preparations have underestimated cellular OXPHOS-capacities, due to the gating effect through a single branch. Complete additivity is defined as the condition when the sum of separately measured respiratory capacities, N + S, is identical to the capacity measured in the state with combined substrates, NS (CI&II). This condition of complete additivity, NS=N+S, would be obtained if electron channeling through supercomplex CI, CIII and CIV does not interact with the pool of redox intermediates in the pathway from CII to CIII and CIV, and if the capacity of the phosphorylation system does not limit OXPHOS capacity (excess E-P capacity factor is zero). In most cases, however, additivity is incomplete, NS < N+S. |
| Adenine nucleotides | AN | Adenine nucleotides, which are also sometimes referred to as adenosines or adenylates, are a group of organic molecules including AMP, ADP and ATP. These molecules present the major players of energy storage and transfer. |
| Aerobic | ox | The aerobic state of metabolism is defined by the presence of oxygen (air) and therefore the potential for oxidative reactions (ox) to proceed, particularly in oxidative phosphorylation (OXPHOS). Aerobic metabolism (with involvement of oxygen) is contrasted with anaerobic metabolism (without involvement of oxygen): Whereas anaerobic metabolism may proceed in the absence or presence of oxygen (anoxic or oxic conditions), aerobic metabolism is restricted to oxic conditions. Below the critical oxygen pressure, aerobic ATP production decreases. |
| Amount of substance | n [mol] | The amount of substance n is a base physical quantity, and the corresponding SI unit is the mole [mol]. Amount of substance (sometimes abbreviated as 'amount' or 'chemical amount') is proportional to the number NX of specified elementary entities X, and the universal proportionality constant is the reciprocal value of the Avogadro constant (SI),
nX = NX·NA-1 nX contained in a system can change due to internal and external transformations, dnX = dinX + denX In the absence of nuclear reactions, the amount of any atom is conserved, e.g., for carbon dinC = 0. This is different for chemical substances or ionic species which are produced or consumed during the advancement of a reaction r, A change in the amount of Xi, dni, in an open system is due to both the internal formation in chemical transformations, drni, and the external transfer, deni, across the system boundaries. dni is positive if Xi is formed as a product of the reaction within the system. deni is negative if Xi flows out of the system and appears as a product in the surroundings (Cohen 2008 IUPAC Green Book). |
| Anaerobic | Anaerobic metabolism takes place without the use of molecular oxygen, in contrast to aerobic metabolism. The capacity for energy assimilation and growth under anoxic conditions is the ultimate criterion for facultative anaerobiosis. Anaerobic metabolism may proceed not only under anoxic conditions or states, but also under hyperoxic and normoxic conditions (aerobic glycolysis), and under hypoxic and microxic conditions below the limiting oxygen pressure. | |
| Anaplerosis | Anaplerosis is the process of formation of intermediates of the tricarboxylic acid cycle. Malic enzyme (mtME), phosphoenolpyruvate carboxykinase (PEPCK), propionyl-CoA carboxylase, pyruvate carboxylase and proline dehydrogenase play important roles in anaplerosis. | |
| Anoxia | anox | Ideally the terms anoxia and anoxic (anox, without oxygen) should be restricted to conditions where molecular oxygen is strictly absent. Practically, effective anoxia is obtained when a further decrease of experimental oxygen levels does not elicit any physiological or biochemical response. The practical definition, therefore, depends on (i) the techiques applied for oxygen removal and minimizing oxygen diffusion into the experimental system, (ii) the sensitivity and limit of detection of analytical methods of measuring oxygen (O2 concentration in the nM range), and (iii) the types of diagnostic tests applied to evaluate effects of trace amounts of oxygen on physiological and biochemical processes. The difficulties involved in defining an absolute limit between anoxic and microxic conditions are best illustrated by a logarithmic scale of oxygen pressure or oxygen concentration. In the anoxic state (State 5), any aerobic type of metabolism cannot take place, whereas anaerobic metabolism may proceed under oxic or anoxic conditions. |
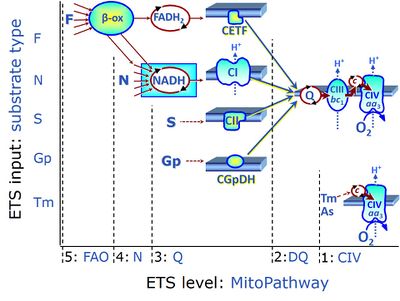
| Ascorbate | As | In respiratory assays for cytochrome c oxidase activity (Complex IV, CIV), ascorbate is added as regenerating system to maintain TMPD in a reduced state. It must be titrated into the respiration medium prior to the addition of TMPD, otherwise the autoxidation reaction velocity is permanently elevated. |
| CHNO-fuel substrate | CHNO | CHNO-fuel substrates are reduced carbon-hydrogen-nitrogen-oxygen substrates which are oxidized in the exergonic process of cell respiration. Mitochondrial pathways are stimulated by CHNO-fuel substrates feeding electrons into the ETS at different levels of integration and in the presence or absence of inhibitors acting on specific enzymes which are gate-keepers and control various pathway segments. |
| Carbohydrate | Carbohydrates, also known as saccharides, are molecules composed of carbon, hydrogen and oxygen. These molecules can be divided by size and complexity into monosaccharides, disaccharides, oligosaccharides, and polysaccharides. Glucose is a monosaccharide considered the primary source of energy in cells and a metabolic intermediate. This carbohydrate undergoes glycolysis, with the generation of pyruvate, that can enter the TCA cycle. Carbohydrates such as glucose and fructose may also be involved in the Crabtree effect. | |
| Carnitine | Car | Carnitine is an important factor for the transport of long-chain fatty acids bound to carnitine (carnitine acyltransferase) into the mitochondrial matrix for subsequent β-oxidation. There are two enantiomers: D- and L-carnitine. Only the L-isomer is physiologically active. |
| Cellular substrates | Ce; Cm | (1) Cellular substrates in vivo, endogenous; Ce.
(2) Cellular substrates in vivo, with exogenous substrate supply from culture medium or serum; Cm.
|
| Citrate | citrate, C6H5O7-3, is a tricarboxylic acid trianion, intermediate of the TCA cycle, obtained by deprotonation of the three carboxy groups of citric acid. Citrate is formed from oxaloacetate and acetyl-CoA through the catalytic activity of the citrate synthase. In the TCA cycle, citrate forms isocitrate by the activity of the aconitase. Citrate can be transported out of the mitochondria by the tricarboxylate transport, situated in the inner mitochondrial membrane. The transport occurs as an antiport of malate from the cytosol and it is a key process for fatty acid and oxaloacetate synthesis in the cytosol. | |
| Coenzyme | A coenzyme or cosubstrate is a cofactor that is attached loosely and transiently to an enzyme, in contrast to a prosthetic group that is attached permanently and tightly. The coenzyme is required by the corresponding enzyme for its activity (IUPAC definition). A coenzyme is 'a low-molecular-weight, non-protein organic compound participating in enzymatic reactions as dissociable acceptor or donor of chemical groups or electrons' (IUPAC definition). | |
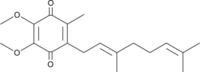
| Coenzyme Q2 | CoQ2 | Coenzyme Q2 or ubiquinone-2 (CoQ2) is a quinone derivate composed of a benzoquinone ring with an isoprenoid side chain consisting of two isoprenoid groups, with two methoxy groups, and with one methyl group. In HRR it is used as a Q-mimetic to detect the redox changes of coenzyme Q at the Q-junction in conjunction with the Q-Module, since the naturally occurring long-chain coenzyme Q (e.g. CoQ10) is trapped within membrane boundaries. CoQ2 can react both with mitochondrial complexes (e.g. CI, CII and CIII) at their quinone-binding sites and with the detecting electrode. |
| Cofactor | A cofactor is 'an organic molecule or ion (usually a metal ion) that is required by an enzyme for its activity. It may be attached either loosely (coenzyme) or tightly (prosthetic group)' (IUPAC definition). | |
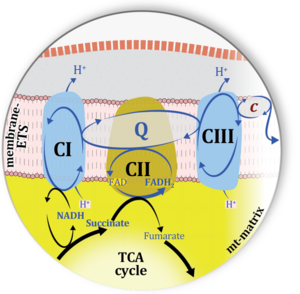
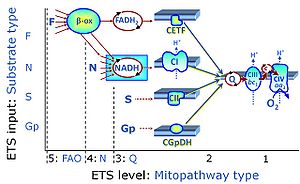
| Complex II ambiguities | CII ambiguities | The current narrative that the reduced coenzymes NADH and FADH2 feed electrons from the tricarboxylic acid (TCA) cycle into the mitochondrial electron transfer system can create ambiguities around respiratory Complex CII. Succinate dehydrogenase or CII reduces FAD to FADH2 in the canonical forward TCA cycle. However, some graphical representations of the membrane-bound electron transfer system (ETS) depict CII as the site of oxidation of FADH2. This leads to the false believe that FADH2 generated by electron transferring flavoprotein (CETF) in fatty acid oxidation and mitochondrial glycerophosphate dehydrogenase (CGpDH) feeds electrons into the ETS through CII. In reality, NADH and succinate produced in the TCA cycle are the substrates of Complexes CI and CII, respectively, and the reduced flavin groups FMNH2 and FADH2 are downstream products of CI and CII, respectively, carrying electrons from CI and CII into the Q-junction. Similarly, CETF and CGpDH feed electrons into the Q-junction but not through CII. The ambiguities surrounding Complex II in the literature call for quality control, to secure scientific standards in current communications on bioenergetics and support adequate clinical applications. |
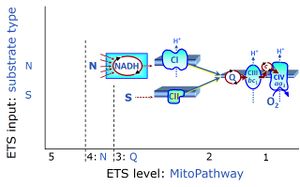
| Convergent electron flow | n.a. | Convergent electron flow is built into the metabolic design of the Electron transfer pathway. The glycolytic pathways are characterized by important divergent branchpoints: phosphoenolpyruvate (PEPCK) branchpoint to pyruvate or oxaloactetate; pyruvate branchpoint to (aerobic) acetyl-CoA or (anaerobic) lactate or alanine. The mitochondrial Electron transfer pathway, in contrast, is characterized by convergent junctions: (1) the N-junction and F-junction in the mitochondrial matrix at ET-pathway level 4, with dehydrogenases (including the TCA cycle) and ß-oxidation generating NADH and FADH2 as substrates for Complex I and electron-transferring flavoprotein complex, respectively, and (2) the Q-junction with inner mt-membrane respiratory complexes at ET-pathway level 3, reducing the oxidized ubiquinone and partially reduced semiquinone to the fully reduced ubiquinol, feeding electrons into Complex III. |
| Creatine | Cr | Creatine is a nitrogenous organic acid that occurs naturally in vertebrates and helps primarily muscle cells to supply energy by increasing the formation of adenosine triphosphate (ATP). |
| Cytochrome c | c | Cytochrome c is a component of the Electron transfer-pathway (Electron transfer pathway) in mitochondria. It is a small heme protein loosely associated with the outer side of the inner mitochondrial membrane. The heme group of cytochrome c transfers electrons from Complex III to Complex IV. The release of cytochrome c into the cytoplasm is associated with apoptosis. Cytochrome c is applied in HRR to test the integrity of the mitochondrial outer membrane (cytochrome c control efficiency). |
| Cytochrome c control efficiency | jcyt c | The cytochrome c control efficiency expresses the control of respiration by externally added cytochrome c, c, as a fractional change of flux from substrate state CHNO to CHNOc. These fluxes are corrected for Rox and may be measured in the OXPHOS state or ET state, but not in the LEAK state. In this flux control efficiency, CHNOc is the reference state with stimulated flux; CHNO is the background state with CHNO substrates, upon which c is added: jcyt c = (JCHNOc-JCHNO)/JCHNOc. |
| Dithionite | Dit Dit - (The abbreviation 'Dith' has been used previously and is stepwise replaced by Dit.) | The sodium salt of Dithionite Na2S2O4 (Dit) is the 'zero oxygen solution powder' used for calibration of oxygen sensors at zero oxygen concentration, or for stepwise reduction of oxygen concentrations in instrumental O2 background tests. It is not recommended to use dithionite in experiments with biological samples or several multisensor approaches, for these see Setting the oxygen concentration. |
| Duroquinol | DQ | ET-pathway level 2 is supported by duroquinol DQ feeding electrons into Complex III (CIII) with further electron transfer to CIV and oxygen. Upstream pathways are inhibited by rotenone and malonic acid in the absence of other substrates linked to ET-pathways with entry into the Q-junction. |
| ET-pathway substrate types | n.a. | See Electron-transfer-pathway state |
| Electron-transfer-pathway state | ET-pathway state |
Electron-transfer-pathway states are obtained in mitochondrial preparations (isolated mitochondria, permeabilized cells, permeabilized tissues, tissue homogenate) by depletion of endogenous substrates and addition to the mitochondrial respiration medium of fuel substrates (CHNO) activating specific mitochondrial pathways, and possibly inhibitors of specific pathways. Mitochondrial electron-transfer-pathway states have to be defined complementary to mitochondrial coupling-control states. Coupling-control states require ET-pathway competent states, including oxygen supply. Categories of SUIT protocols are defined according to mitochondrial ET-pathway states. » MiPNet article |
| Energy charge | AEC | The energy charge of the adenylate system or adenylate energy charge (AEC) has been defined by Atkinson and Walton (1967) as (ATP + ½ ADP)/(AMP + ADP + ATP). Wheather the AEC is a fundamental metabolic control parameter remains a controversial topic. |
| Ethanol | ethanol abs. |
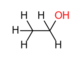
Ethanol or ethyl alcohol, C2H6O or EtOH, is widely used in the laboratory, particularly as a solvent and cleaning agent. There are different grades of high purity ethanol. Up to a purity of 95.6 % ethanol can be separated from water by destillation. Higher concentrations than 95% require usage of additives that disrupt the azeotrope composition and allow further distillation. Ethanol is qualified as "absolute" if it contains no more than one percent water. Whenever 'ethanol abs.' is mentioned without further specification in published protocols, it refers to ≥ 99 % ethanol a.r. (analytical reagent grade).
|
| FADH2 | FADH2 | FADH2 and FAD: see Flavin adenine dinucleotide. |
| Fatty acid | FA | Fatty acids are carboxylic acids with a carbon aliphatic chain. The fatty acids can be divided by the length of this chain, being considered as short-chain (1–6 carbons), medium-chain (7–12 carbons) and long-chain and very long-chain fatty acids (>12 carbons).
Long-chain fatty acids must be bound to carnitine to enter the mitochondrial matrix, in a reaction that can be catalysed by carnitine acyltransferase. For this reason, long-chain fatty acids, such as palmitate (16 carbons) is frequently supplied to mt-preparations in the activated form of palmitoylcarnitine. Fatty acids with shorter chains, as octanoate (8 carbons) may enter the mitochondrial matrix, however, in HRR they are more frequently supplied also in the activated form, such as octanoylcarnitine. Once in the mitochondrial matrix, the fatty acid oxidation (FAO) occurs, generating acetyl-CoA, NADH and FADH2. In the fatty acid oxidation pathway control state electrons are fed into the F-junction involving the electron transferring flavoprotein (CETF). FAO cannot proceed without a substrate combination of fatty acids & malate, and inhibition of CI blocks FAO. Low concentration of malate, typically 0.1 mM, does not saturate the N-pathway; but saturates the F-pathway. |
| Fatty acid oxidation | FAO | Fatty acid oxidation is a multi-step process by which fatty acids are broken down in β-oxidation to generate acetyl-CoA, NADH and FADH2 for further electron transfer to CoQ. Whereas NADH is the substrate of CI, FADH2 is the substrate of electron-transferring flavoprotein complex (CETF) which is localized on the matrix face of the mtIM, and supplies electrons from FADH2 to CoQ. Before the ß-oxidation in the mitochondrial matrix, fatty acids (short-chain with 1-6, medium-chain with 7–12, long-chain with >12 carbon atoms) are activated by fatty acyl-CoA synthases (thiokinases) in the cytosol. For the mitochondrial transport of long-chain fatty acids the mtOM-enzyme carnitine palmitoyltransferase I (CPT-1; considered as a rate-limiting step in FAO) is required which generates an acyl-carnitine intermediate from acyl-CoA and carnitine. In the next step, an integral mtIM protein carnitine-acylcarnitine translocase (CACT) catalyzes the entrance of acyl-carnitines into the mitochondrial matrix in exchange for free carnitines. In the inner side of the mtIM, another enzyme carnitine palmitoyltransferase 2 (CPT-2) converts the acyl-carnitines to carnitine and acyl-CoAs, which undergo ß-oxidation in the mitochondrial matrix. Short- and medium-chain fatty acids do not require the carnitine shuttle for mitochondrial transport. Octanoate, but not palmitate, (eight- and 16-carbon saturated fatty acids) may pass the mt-membranes, but both are frequently supplied to mt-preparations in the activated form of octanoylcarnitine or palmitoylcarnitine. |
| Flavin adenine dinucleotide | FAD, FADH2 | Flavin adenine dinucleotide, FAD and FADH2, is an oxidation-reduction prosthetic group (redox cofactor; compare NADH). FMN and FAD are the prosthetic groups of flavoproteins (flavin dehydrogenases). Type F substrates (fatty acids) generate FADH2, the substrate of electron transferring flavoprotein (CETF). Thus FADH2 forms a junction or funnel of electron transfer to CETF, the F-junction (compare N-junction, Q-junction), in the F-pathway control state. In contrast, FADH2 is not the substrate but the internal product of succinate dehydrogenase (CII). FAD is the oxidized (quinone) form, which is reduced to FADH2 (hydroquinone form) by accepting two electrons and two protons. |
| Free radicals | A free radical is any atom or molecule that contains one or more unpaired electrons in an orbital. The degree of chemical reactivity depends on the localization of unpaired electrons. Free radicals are extremely reactive, and they can either donate or accept an electron from other molecules. Free radicals that include oxygen radicals and derivatives of oxygen are reactive oxygen species (ROS). Likewise, reactive nitrogen species (RNS) are nitric oxide-derived compounds. ROS/RNS include oxygen/nitrogen free radicals and non-radicals that are easily converted into radicals. Mitochondria are a main endogenous source of free radicals in cells and consequently are exposed to oxidative-nitrosative damage. Electron transfer in the electron transfer-pathway (ET-pathway) is not perfect, leading an electron leakage. This electron leakage permits the formation of ROS such as superoxide anion (O2•−), hydrogen peroxide (H2O2) and the hydroxyl radical (HO•). | |
| Glucose | Glc | Glucose, also known as D-glucose or dextrose, is a monosaccharide and an important carbohydrate in biology. Cells use it as the primary source of energy and a metabolic intermediate. |
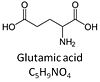
| Glutamate | G | Glutamic acid, C5H9NO4, is an amino acid which occurs under physiological conditions mainly as the anion glutamate-, G, with pKa1 = 2.1, pKa2 = 4.07 and pKa3 = 9.47. Glutamate&malate is a substrate combination supporting an N-linked pathway control state, when glutamate is transported into the mt-matrix via the glutamate-aspartate carrier and reacts with oxaloacetate in the transaminase reaction to form aspartate and oxoglutarate. Glutamate as the sole substrate is transported by the electroneutral glutamate-/OH- exchanger, and is oxidized in the mitochondrial matrix by glutamate dehydrogenase to α-ketoglutarate (2-oxoglutarate), representing the glutamate-anaplerotic pathway control state. Ammonia (the byproduct of the reaction) passes freely through the mitochondrial membrane. |
| Glutamate-aspartate carrier | The glutamate-aspartate carrier catalyzes the electrogenic antiport of glutamate- +H+ for aspartate-. It is an important component of the malate-aspartate shuttle in many mitochondria. Due to the symport of glutamate- + +H+, the glutamate-aspartate antiport is not electroneutal and may be impaired by uncoupling. Aminooxyacetate is an inhibitor of the glutamate-aspartate carrier. | |
| Glycerophosphate | Gp | Glycerophosphate (synonym: α-glycerophosphate; glycerol-3-phosphate; C3H9O6P) is an organophosphate and it is a component of glycerophospholipids. The mitochondrial Glycerophosphate dehydrogenase Complex oxidizes glycerophosphate to dihydroxyacetone phosphate and feeds electrons directly to ubiquinone. |
| Hydride | H- | The hydride anion is the species H−. |
| Hydrogen | H2 | Molecular hydrogen H2 is a constituent of the air with a volume fraction of 0.00005. It is a colorless and odorless gas with a molecular mass of 2.016. Its pharmacological potential and effects on mitochondrial metabolism are discussed in various publications without complete evidence on the underlying mechanisms. |
| Hydrogen ion | H+ | The terms hydrogen ion H+ and proton, p or p+, are used synonymously in chemistry. A hydrogen ion is a positively charged molecule. In particle physics, however, a proton is a submolecular and subatomic particle with a positive electric charge. The H+ ion has no electrons and is a bare charge with only about 1/64 000 of the radius of a hydrogen atom. Free H+ is extremely reactive, with an extremely short lifetime in aqueous solutions. There H+ forms the hydronium ion H3O+, which in turn is further solvated by water molecules in clusters such as H5O2+ and H9O4+. The transfer of H+ in an acid–base reaction is referred to as proton transfer. The acid is the H+ donor and the base is the H+ acceptor. |
| Hydrogen peroxide | H2O2 | Hydrogen peroxide, H2O2 or dihydrogen dioxide, is one of several reactive oxygen intermediates generally referred to as reactive oxygen species (ROS). It is formed in various enzyme-catalyzed reactions (e.g., superoxide dismutase) with the potential to damage cellular molecules and structures. H2O2 is dismutated by catalase to water and oxygen. H2O2 is produced as a signaling molecule in aerobic metabolism and passes membranes more easily compared to other ROS. |
| Hydrogen sulfide | H2S | Hydrogen sulfide (H2S) is involved in signaling and may have have further biological importance. |
| Hydron | H+ | Hydron is the general name for the cation H+ used without regard to the nuclear mass of the hydrogen entity (H is the hydro group), either for H in its natural abundance or without distinction between the isotopes. |
| Hydronium ion | H3O+ | H+ forms the hydronium ion H3O+, which in turn is further solvated by water molecules in clusters such as H5O2+ and H9O4+. |
| Hydroxybutyrate | β-hydroxybutyrate or 3-hydroxybutyrate is a ketone body that can be used as a NADH-linked substrate. The β-hydroxybutyrate dehydrogenase produces acetoacetate while reducing NAD+ to NADH.
| |
| Inorganic phosphate | Pi | Inorgnic phosphate (Pi) is a salt of phosphoric acid. In solution near physiological pH, the species HPO42- and H2PO4- dominate. See also: Phosphate carrier (Pic). |
| Isocitrate | isocitrate, C6H5O7-3, is a tricarboxylic acid trianion, intermediate of the TCA cycle, obtained by isomerization of citrate. The process is catalyzed by aconitase, forming the enzyme-bound intermediate cis-aconitate. | |
| Jmax | Jmax | Jmax is the maximum pathway flux (e.g. oxygen flux) obtained at saturating substrate concentration. Jmax is a function of metabolic state. In hyperbolic ADP or oxygen kinetics, Jmax is calculated by extrapolation of the hyperbolic function, with good agreement between the calculated and directly measured fluxes, when substrate levels are >20 times the c50 or p50. |
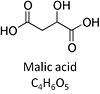
| Malate | M |
Malic acid, C4H6O5, occurs under physiological conditions as the anion malate2-, M, with pKa1 = 3.40 and pKa2 = 5.20. L-Malate is formed from fumarate in the TCA cycle in the mitochondrial matrix, where it is the substrate of malate dehydrogenase oxidized to oxaloacetate. Malate is also formed in the cytosol. It cannot permeate through the lipid bilayer of membranes and hence requires a carrier (dicarboxylate carrier, tricarboxylate carrier and 2-oxoglutarate carrier). Malate alone cannot support respiration of mt-preparations from most tissues, since oxaloacetate accumulates in the absence of pyruvate or glutamate. Malate is a type N substrate (N) required for the FAO-pathway. In the presence of anaplerotic pathways (e.g., mitochondrial malic enzyme, mtME) the capacity of the FAO-pathway can be overestimated due to a contribution of NADH-linked respiration, F(N) (see SUIT-002). |
| Malate transport | Carriers for malate: | |
| Metabolic control variable | X | A metabolic control variable X causes the transition between a background state Y (background rate YX) and a reference state Z (reference rate ZX). X may be a stimulator or activator of flux, inducing the step change from background to reference steady state (Y to Z). Alternatively, X may be an inhibitor of flux, absent in the reference state but present in the background state (step change from Z to Y). |
| Methylmalonic acid | Mma | Methylmalonic acid (Mma) is a common intermediate in many catabolic processes. In methylmalonic acidemia mitochondrial dysfunction can be observed, related to accumulation of Mma and associated with neurological symptoms. |
| MitoKit-CII | Cell permeable prodrugs, composed of MitoKit-CII/Succinate-nv and MitoKit-CII/Malonate-nv, stimulates (Snv) or inhibits (Mnanv) mitochondrial respiration in CI-deficient human blood cells, fibroblasts and heart fibres, acting on Complex II of the electron transfer system. | |
| NS-pathway control state | NS, CI&II | NS-pathway control is exerted in the NS-linked substrate state (flux in the NS-linked substrate state, NS; or Complex I&II, CI&II-linked substrate state). NS-OXPHOS capacity provides an estimate of physiologically relevant maximum mitochondrial respiratory capacity. NS is induced in mt-preparations by addition of NADH-generating substrates (N-pathway control state in combination with succinate (Succinate pathway; S). Whereas NS expresses substrate control in terms of substrate types (N and S), CI&II defines the same concept in terms of convergent electron transfer to the Q-junction (pathway control). NS is the abbreviation for the combination of NADH-linked substrates (N) and succinate (S). This physiological substrate combination is required for partial reconstitution of TCA cycle function and convergent electron-input into the Q-junction, to compensate for metabolite depletion into the incubation medium. NS in combination exerts an additive effect of convergent electron flow in most types of mitochondria. |
| Nicotinamide adenine dinucleotide | NADH | Nicotinamide adenine dinucleotide, NAD+ and NADH (pyridine nucleotide coenzymes, NAD and NADP), is an oxidation-reduction coenzyme (redox cofactor; compare FADH2). In the NADH electron transfer-pathway state fuelled by type N-substrates, mt-matrix dehydrogenases generate NADH, the substrate of Complex I (CI). The reduced N-substrate RH2 is oxidized and NAD+ is reduced to NADH,:::: RH2 + NAD+ → NADH + H+ + R. The mt-NADH pool integrates the activity of the TCA cycle and various matrix dehydrogenases upstream of CI, and thus forms a junction or funnel of electron transfer to CI, the N-junction (compare F-junction, Q-junction). NAD+ and NADH are not permeable through the mt-inner membrane, mtIM. Therefore, an increase of mitochondrial respiration after the addition of NADH may indicate an alteration of the mtIM integrity. Cytosolic NADH is effectively made available for mitochondrial respiration through the malate-aspartate shuttle or glycerophosphate dehydrogenase Complex. |
| Octanoate | Oca | Octanoate (octanoic acid). C8H16O2 Common name: Caprylic acid. |
| Octanoylcarnitine | Oct | Octanoylcarnitine is a medium-chain fatty acid (octanoic acid: eight-carbon saturated fatty acid) covalently linked to carnitine, frequently applied as a substrate for fatty acid oxidation (FAO) in mitochondrial preparations. |
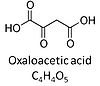
| Oxaloacetate | Oa | Oxaloacetic acid, C4H4O5, occurs under physiological conditions as the anion oxaloacetate2-, Oa. Oxaloacetate is formed from malate by MDH. Oa reacts with acetyl-CoA through citrate synthase to form citrate, or with glutamate through transaminase to form oxoglutarate and aspartate. Oa transport is restricted across the inner mt-membrane of various tissues. Oa is a potent inhibitor of succinate dehydrogenase. |
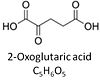
| Oxoglutarate | Og | 2-Oxoglutaric acid or alpha-ketoglutaric acid, C5H6O5, occurs under physiological conditions as the anion 2-Oxoglutarate2-, Og. 2-Oxoglutarate (alpha-ketoglutarate) is formed from isocitrate as a product of isocitrate dehydrogenase (IDH) in the TCA cycle, and is a substrate of oxoglutarate dehydrogenase (OgDH). The 2-oxoglutarate carrier exchanges malate2- for 2-oxoglutarate2- as part of the malate-aspartate shuttle. In the cytosol, oxoglutarate+aspartate are transaminated to form oxaloacetate+glutamate. Cytosolic malate dehydrogenase converts oxaloacetate+NADH to malate. |
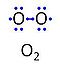
| Oxygen | O2 | Molecular oxygen, O2 or dioxygen, has two atoms of oxygen, O, which is the chemical element with atomic number 8. The relative molecular mass of O2, Mr,O2, is 32 (or 31.9988). The element O has 8 protons, 8 neutrons and 8 electrons. In the figure, the two electrons in the first electron shell are not shown. Of the six electrons in the outer shell (blue bullets), one electron from each of the two atoms is shared in O2 forming the covalent bond, and one electron in each atom is unpaired. |
| P50 | p50 | p50 is the oxygen partial pressure at which (a) respiratory flux is 50% of maximum oxygen flux, Jmax, at saturating oxygen levels. The oxygen affinity is indirectly proportional to the p50. The p50 depends on metabolic state and rate. (b) p50 is the oxygen partial pressure at which oxygen binding (on myoglobin, haemoglobin) is 50%, or desaturation is 50%. |
| Palmitate | Paa | Palmitate is a term for the salts and esters of palmitic acid (CH3(CH2)14COOH). Palmitic acid is the first fatty acid produced during fatty acid synthesis and the precursor to longer fatty acids. Palmitate negatively feeds back on acetyl-CoA carboxylase (ACC), which is responsible for converting acetyl-CoA to malonyl-CoA, which in turn is used to add to the growing acyl chain, thus preventing further palmitate generation. In order to dissolve the water-insoluble sodium palmitate, BSA is needed to form the water-soluble compound called palmitate:BSA. |
| Palmitoyl-CoA | Pa-CoA | Palmitoyl-CoA is a coenzyme A derivative of palmitate formed by acyl-CoA synthase. In contrast to medium- and short-chain acyl-CoA, palmitoyl-CoA cannot freely diffuse into the mitochondrial matrix. Formation of palmitoylcarnitine by CPTI is necessary prior to transfer into mitochondria for further fatty acid oxidation (β-oxidation). To study Fatty acid oxidation using Palmitoyl-CoA, Carnitine and low amount of malate is needed on mitochondrial preparations. |
| Palmitoylcarnitine | Pal | Palmitoylcarnitine is an ester derivative of carnitine (long-chain acylcarnitine) involved in the metabolism of fatty acids. Within the cell, palmitoylcarnitine is transported into the mitochondria to deliver palmitate for fatty acid oxidation and energy production. |
| Phosphate | Pi | See: Inorganic phosphate |
| Phosphocreatine | PCr | Phosphocreatine is a high energy compound in the skeletal muscle of vertebrates and is present in 4 to 5 times the concentration of ATP. |
| Preparation of SUIT chemicals | Preparation of SUIT chemicals describes the preparation of chemicals used in Substrate-Uncoupler-Inhibitor Ttitration (SUIT) protocols. | |
| Product | Chemistry: A product in a chemical reaction has a positive stoichiometric number since it is produced, whereas a substrate has a negative stoichiometric number since it is consumed. QM: A product is the result of a process. The ISO 9000 definition of “product” includes services as a product category, so that it is no longer correct to refer to “products and services”. | |
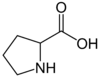
| Proline | Pro |
Proline (Pro), C5H9NO2, is an amino acid which occurs under physiological conditions mainly in the nonpolar form, with pKa1 = 1.99 pKa2 = 10.96. Proline is an anaplerotic substrate that supports both the proline pathway control state and the glutamate-anaplerotic pathway control state. Proline is used as a single substrate or in combination with carbohydrate-derived metabolites in mitochondria particularly of flight muscle of many (but not all) insects. Proline is oxidized to delta-1-pyrroline-5-carboxylate by the mtIM L-proline:quinone oxidoreductase (proline dehydrogenase, ProDH), with reduction of FAD to FADH2 and direct entry into the Q-junction. delta-1-pyrroline-5-carboxylate is converted to glutamate by 1-pyrroline-5-carboxylate dehydrogenase. |
| Prosthetic group | A prosthetic group is a cofactor that is attached permanently and tightly or even covalently to an enzyme and that is regenerated in each enzymatic turnover. Thus a prostethic group is distinguished from a coenzyme or cosubstrate that is attached loosely and transiently. Like a coenzyme, the prosthetic group is required by an enzyme for its activity. A prosthetic group is 'a tightly bound, specific nonpolypeptide unit in a protein determining and involved in its biological activity' (IUPAC definition). FMN/FMNH2 and FAD/FADH2 are prosthetic groups of Complex I and Complex II, respectively. | |
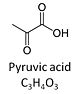
| Pyruvate | P | Pyruvic acid, C3H4O3, is an alpha-keto monocarboxylic acid which occurs under physiological conditions mainly as the anion pyruvate-, P, with pKa = 2.5. Pyruvate is formed in glycolysis from phosphoenolpyruvate. In the cytosol, pyruvate is a substrate of lactate dehydrogenase. Pyruvate enters the mitochondrial matrix via a specific low Km' H+/monocarboxylate cotransporter known as the pyruvate carrier. Similarly, the plasma membrane of many cell types has H+/monocarboxylate cotransporter activity and pyruvate can thus be added as a substrate to living cells. In the mt-matrix the oxidative decarboxylation of pyruvate is catalyzed by pyruvate dehydrogenase and yields acetyl-CoA. Pyruvate competitively reverses the inhibition of cytochrome c oxidase by cyanide. Pyruvate is an antioxidant reacting with hydrogen peroxide. |
| Q-pools | Q | Different Q-pools are more or less clearly distinguished in the cell, related to a variety of models describing degress of Q-pool behavior. (1) CoQ-pools are distinguished according to their compartmentation in the cell: mitochondrial CoQ (mtCoQ) and CoQ in other organelles versus plasma-membrane CoQ. (2) The total mitochondrial CoQ-pool mtCoQ is partitioned into an ETS-reactive Q-pool, Qra, and an inactive mtCoQ-pool, Qia. (2a) The Qra-pool is fully reduced in the form of quinol QH2 under anoxia, and fully oxidized in the form of quinone in aerobic mitochondrial preparations incubated without CHNO-fuel substrates. Intermediate redox states of Qra are sensitive to pathway control and coupling control of mitochondrial electron transfer and OXPHOS. (2b) The Qia-pool remains partially reduced and oxidized independent of aerobic-anoxic transitions. The redox state of Qia is insensitive to changes in mitochondrial respiratory states. (3) The Qra-pool is partitioned into Q with Q-pool behavior according to the fluid-state model (synonymous: random-collision model) and Q tightly bound to supercomplexes according to the solid-state model. The two models describe the extremes in a continuum of homogenous or heterogenous Q-pool behavior. The CII-Q-CIII segment of the S-pathway is frequently considered to follow homogenous Q-pool behavior participating in the Qhom-pool, whereas the CI-Q-CIII segment of the N-pathway indicates supercomplex organization and metabolic channeling with different degrees of Q-pool heterogeneity contributing to the Qhet-pool. |
| Reactive nitrogen species | RNS | Reactive nitrogen species, RNS, are nitric oxide-derived oxidants. The main source of RNS is nitric oxide (NO•). NO• plays an important role in cell signaling and in oxidative-nitrosative stress. |
| Reactive oxygen species | ROS | Reactive oxygen species, ROS, are molecules derived from molecular oxygen, including free oxygen radicals, which are more reactive than O2. Physiologically and pathologically important ROS include superoxide, the hydroxyl radical and hydroxide ion, hydrogen peroxide and other peroxides. These are important in cell signalling, oxidative defence mechanisms and oxidative stress. |
| Substrate | IUPAC distinguishes three definitions of 'substrate': (1) The chemical entity whose conversion to a product or products is catalysed by one or several enzymes. (2) A solution or dry mixture containing all ingredients which are necessary for the growth of a microbial culture or for product formation. (3) Component in the nutrient medium, supplying the organisms with carbon (C-substrate), nitrogen (N-substrate), etc. A substrate in a chemical reaction has a negative stoichiometric number since it is consumed, whereas a product has a positive stoichiometric number since it is produced. | |
| Substrate-uncoupler-inhibitor titration | SUIT | Mitochondrial Substrate-uncoupler-inhibitor titration (SUIT) protocols are used with mitochondrial preparations to study respiratory control in a sequence of coupling and substrates states induced by multiple titrations within a single experimental assay. |
| Substrates as electron donors | Sred | Substrates as electron donors are reduced fuel compounds Sred that are oxidized to an oxidized product Pox during H+-linked electron transfer, Sred → Pox + 2{H+ + e-}. Mitochondrial respiration depends on a continuous flow of electron-supplying substrates across the mitochondrial membranes into the matrix space. Many substrates are strong anions that cannot permeate lipid membranes and hence require carriers. |
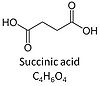
| Succinate | S | Succinic acid, C4H6O4, (butanedioic acid) is a dicarboxylic acid which occurs under physiological conditions as the anion succinate2-, S, with pKa1 = 4.2 and pKa2 = 5.6. Succinate is formed in the TCA cycle, and is a substrate of CII, reacting to fumarate and feeding electrons into the Q-junction. Succinate (CII-linked) and NADH (CI-linked) provide convergent electron entries into the Q-junction. Succinate is transported across the inner mt-membrane by the dicarboxylate carrier. The plasma membrane of many cell types is impermeable for succinate (but see Zhunussova 2015 Am J Cancer Res for an exception). Incubation of mt-preparations by succinate alone may lead to accumulation of oxaloacetate, which is a potent inhibitor of Complex II (compare Succinate and rotenone). High activities of mt-Malic enzyme (mtME) prevent accumulation of oxaloacetate in incubations with succinate without rotenone. |
| Succinate transport | The dicarboxylate carrier catalyses the electroneutral exchange of succinate2- for HPO4-2-. | |
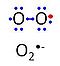
| Superoxide | O2•- | 'Superoxide anion, O2•-, is a free radical formed in a one-electron reduction of molecular oxygen (red bullet in the figure), yielding a negatively charged molecule with a single unpaired electron (blue bullet on the left). It is highly reactive with organic compounds, and its intracellular concentration is kept under control by superoxide dismutase. |
| TMPD | Tm | N,N,N',N-Tetramethyl-p-phenylenediamine dihydrochloride, TMPD, is applied as an artificial substrate for reducing cytochrome c in the respirometric assay for cytochrome c oxidase (CIV) activity. It is maintained in a reduced state by ascorbate and undergoes autoxidation as a function of oxygen pressure, TMPD, ascorbate and cytochrome c concentration. |
| Taurine | Taurine, or 2-Aminoethan sulfonic acid, is one of the most abundant low-molecular-weight organic constituents in animals and humans. It has a multitude of functions in different types of tissue, one of which is the stabilization of membranes. Because of this and its antioxidative effect, taurine is a component of the respiration media MiR05 and MiR06 to preserve mitochondrial function. | |
| Tetrahydrofolate | THF | Tetrahydrofolate, THF, is the substrate in mitochondrial folate-mediated 1C metabolism, an NADH-linked pathway leading to the formation of formate which is exported to the cytosol. |
| Volume of the solute | Most of the chemicals for SUIT protocol titrations are prepared by weighing the substance on the balance, transferring to a volumetric glass flask and adding solvent until the intended volume is reached. However, for practical reasons some of the chemical compounds are prepared by just adding the solvent instead of adjusting it's volume. For example, this approach is useful if the substance is very toxic. Then an arbitratry amount is taken, its mass determined on the balance without trying to reach a specific value and the necessary amount of solvent is added. Adding the solvent instead of adjusting its volume is also useful if small amounts are needed (e.g. 1 mL) or if the compound has to be prepared directly before using it like Pyruvate. In these cases the volume contributed by the solute was tested. | |
| Water | H2O | Water, H2O, is widely used in the laboratory, particularly as a solvent and cleaning agent. Chemically pure water is prepared in various grades of purification: double distilled water (ddH2O) versus distilled water (dH2O or aqua destillata, a.d.) and deionized or demineralized water (diH2O) with various combination purification methods. When H2O is mentioned without further specification in published protocols, it is frequently assumed that the standards of each laboratory are applied as to the quality of purified water. Purification is not only to be controlled with respect to salt content and corresponding electrical conductivity (ultra-pure water: 5.5 μS/m due to H+ and OH- ions), but also in terms of microbial contamination. |