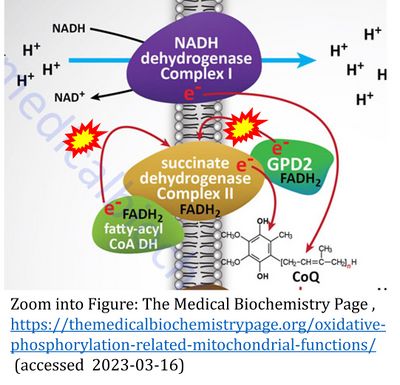
Description
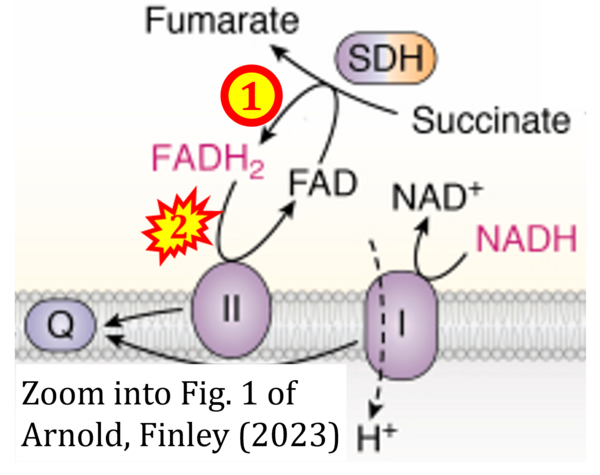
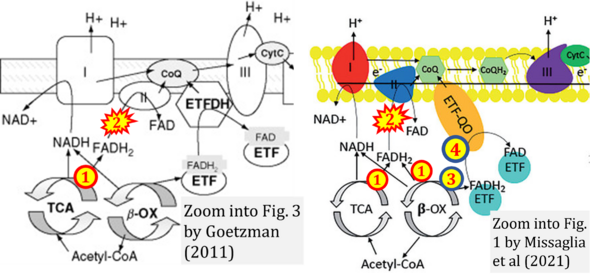
Two ambiguities or misconceptions around respiratory Complex II (CII) have their roots in the narrative that reduced coenzymes (NADH and FADH2) feed electrons from the tricarboxylic acid (TCA) cycle into the mitochondrial electron transfer system. In graphical representations propagating the first ambiguity, succinate dehydrogenase or CII in the canonical (forward) TCA cycle is shown to reduce FAD to FADH2 (correct), yet CII in the membrane-bound electron transfer system (ETS) is paradoxically represented as the site of oxidation of FADH2 to FAD. With minor expansion of the tale on electron transfer from FADH2 into CII, we arrive at the misconception that FADH2 generated by electron transferring flavoprotein (CETF) in fatty acid oxidation and by mitochondrial glycerophosphate dehydrogenase (CGpDH) feeds electrons into the ETS through CII. For clarification, recall that NADH and succinate formed in the TCA cycle in the mitochondrial matrix are the upstream substrates of Complexes CI and CII, whereas the reduced flavin groups FMNH2 of flavin mononucleotide and FADH2 of flavin adenine dinucleotide are products of CI and CII, respectively, with downstream electron flow from CI and CII into the Q-junction. CETF and CGpDH feed electrons into the Q-junction convergent with but not through CII into the Q-junction. Numerous Complex II ambiguities in the literature with discrepancies in graphical representations and text require quality control to secure scientific standards in current communications on bioenergetics.
Abbreviation: CII ambiguities
Communicated by Gnaiger E (2023-03-03) last update 2023-03-18
Is there a problem ?
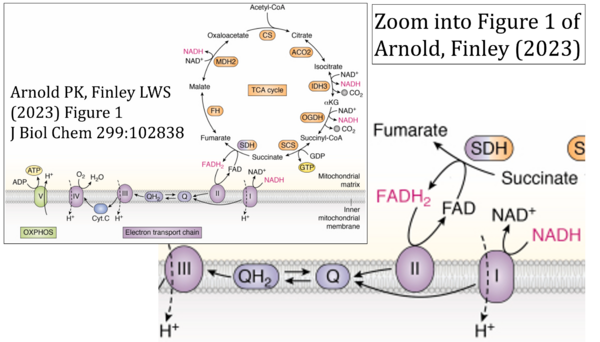
- Ref. [1] Arnold PK, Finley LWS (2022) Regulation and function of the mammalian tricarboxylic acid cycle. J Biol Chem 299:102838. - »Bioblast link«
FADH2 and FMNH2 in the S- and N-pathways
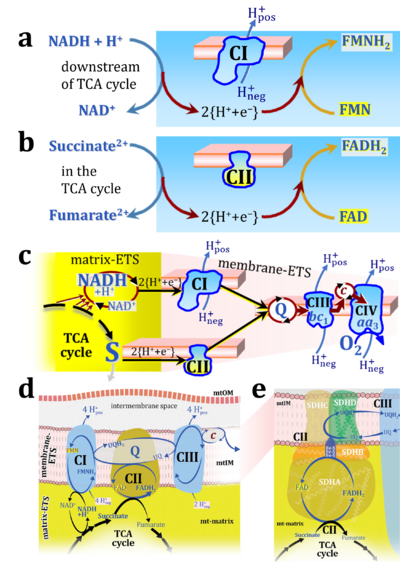
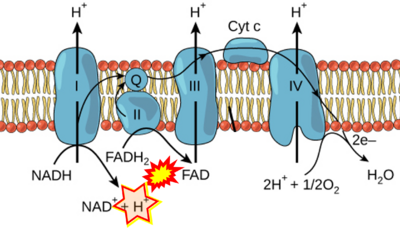
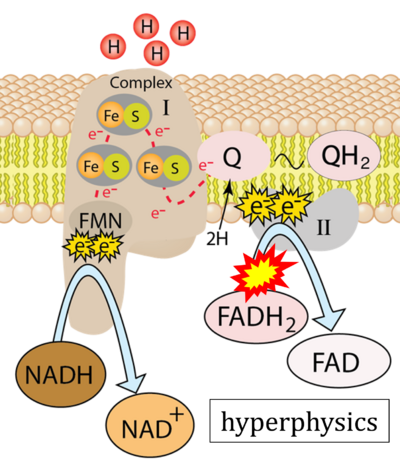
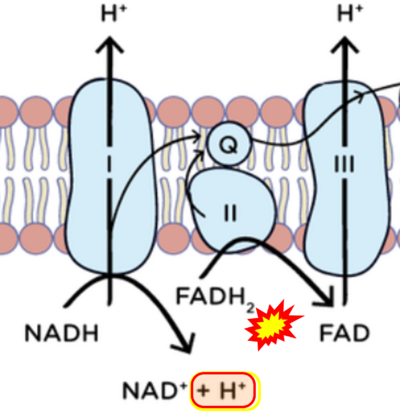
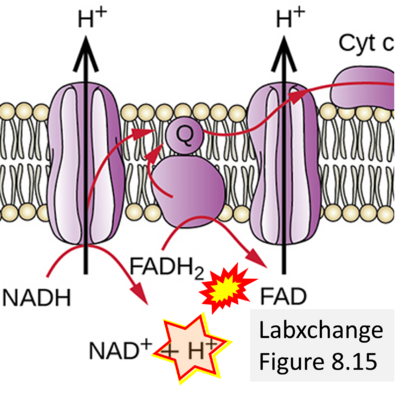
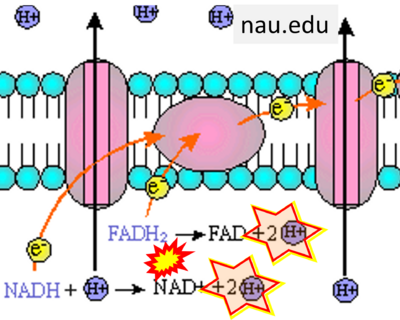
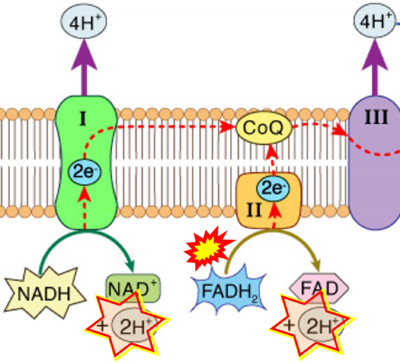
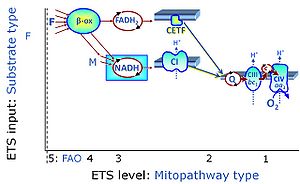
- Respiratory Complex CII participates both in the membrane-bound electron transfer system (membrane-ETS) and TCA cycle (matrix-ETS plus CII; Gnaiger et al 2020). Branches of electron transfer from the reduced coenzyme NADH of nicotinamide adenine dinucleotide N and succinate S converge at the Q-junction in the ETS (Figure a; modified from Gnaiger 2020).
- The reduced flavin groups FADH2 of flavin adenine dinucleotide and FMNH2 of flavin mononucleotide are at functionally comparable levels in the electron transfer to Q from CII and CI, respectively, just as succinate and NADH are the comparable reduced substrates of CII and CI, respectively (Gnaiger 2020). In CII the oxidized form FAD is reduced by succinate to the product FADH2 and the oxidized product fumarate in the TCA cycle. In CI FMN is reduced by NADH forming FMNH2 and the oxidized NAD+. FADH2 and FMNH2 are reoxidized downstream in CII and CI by electron transfer to Q in the membrane-bound ETS (Figure b).
- Ref. [2] Gnaiger E (2020) Mitochondrial pathways and respiratory control. An introduction to OXPHOS analysis. 5th ed. https://doi.org/10.26124/bec:2020-0002
- Ref. [3] Gnaiger E et al ― MitoEAGLE Task Group (2020) Mitochondrial physiology. https://doi.org/10.26124/bec:2020-0001.v1
The source and consequence of ambiguities
- Ambiguities emerge if the presentation of a concept is vague to an extent that allows for equivocal interpretations. As a consequence of ambiguous representations, even a basically clear and quite simple concept may be communicated further without appropriate reflection as an erroneous divergence from an established truth. The following quotes from Cooper (2000) provide an example.
- Ref. [4] Cooper GM (2000) The cell: a molecular approach. 2nd edition. Sunderland (MA): Sinauer Associates Available from: https://www.ncbi.nlm.nih.gov/books/NBK9885/ - »Bioblast link«
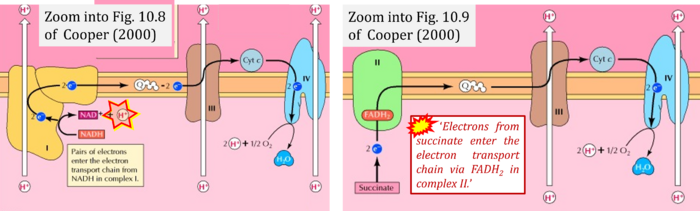
- (1) 'Electrons from NADH enter the electron transport chain in complex I, .. A distinct protein complex (complex II), which consists of four polypeptides, receives electrons from the citric acid cycle intermediate, succinate (Figure 10.9). These electrons are transferred to FADH2, rather than to NADH, and then to coenzyme Q.'
- Comment: Here, the frequent comparison is made between FADH2 (linked to CII) and NADH (linked to CI).
- (2) 'In contrast to the transfer of electrons from NADH to coenzyme Q at complex I, the transfer of electrons from FADH2 to coenzyme Q is not associated with a significant decrease in free energy and, therefore, is not coupled to ATP synthesis.'
- Comment: Note that CI is in the path of the transfer of electrons from NADH to coenzyme Q. In contrast, the transfer of electrons from FADH2 to coenzyme Q is downstream of CII. Thus even a large Gibbs force ('decrease in free energy') in FADH2→Q would fail to drive the coupled process of proton translocation through CII, since the Gibbs force in S→FADH2 is missing. (In parentheses: None of these steps are coupled to ATP synthesis. Redox-driven proton translocation should not be confused with pmF-driven phosphorylation of ADP).
- (2) 'In contrast to the transfer of electrons from NADH to coenzyme Q at complex I, the transfer of electrons from FADH2 to coenzyme Q is not associated with a significant decrease in free energy and, therefore, is not coupled to ATP synthesis.'
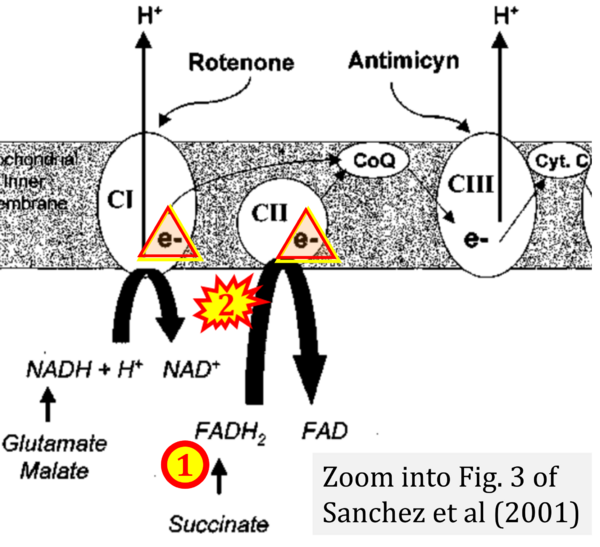
- (3) 'Electrons from succinate enter the electron transport chain via FADH2 in complex II. They are then transferred to coenzyme Q and carried through the rest of the electron transport chain ..'
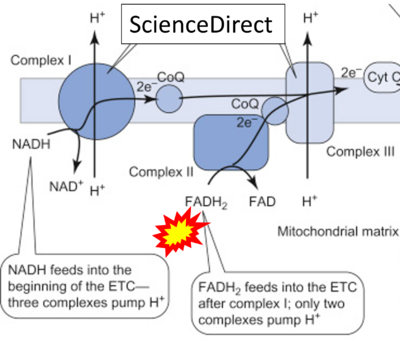
- Comment: The ambiguity is caused by a lack of unequivocal definition of the electron transfer system ('electron transport chain'). CII receives electrons (1) from succinate, yet it is suggested that electrons (from succinate) enter the electron transport chain (3) via FADH2 in complex II. Then two contrasting definitions are implied of the term 'electron transport chain' or better membrane-bound electron transfer system, membrane-ETS. (a) If CII is part of the membrane-ETS, then electrons enter the membrane-ETS from succinate (1) but not from FADH2. (b) If electrons enter the 'electron transport chain' via FADH2 in Complex II (3), then CII would be upstream and hence not part of the membrane-ETS (to which conclusion, obviously - see Figure - nobody would agree). Dismissing concept (b) of the membrane-ETS, then remains the ambiguity, if electrons enter the membrane-ETS from FADH2 (3, wrong) or from succinate (1, correct).
FADH2 - FAD confusion in the S-pathway
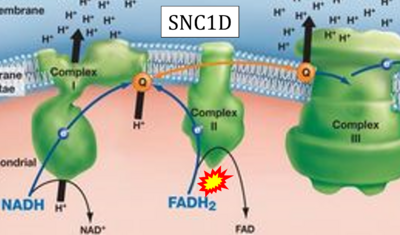
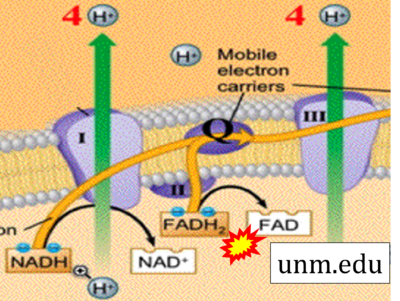
- FADH2 appears in several publications as the substrate of CII in the electron transfer system linked to succinate oxidation. It is surprising that this error is widely propagated particularly in the most recent literature. For clarification, see Gnaiger (2020) page 48.
- The following examples are listed chronologically and illustrate
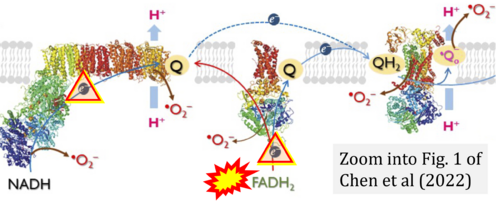
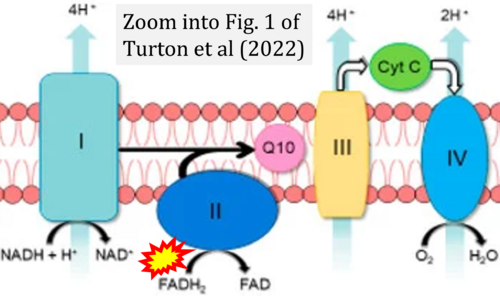
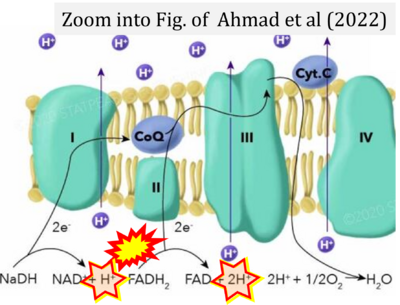
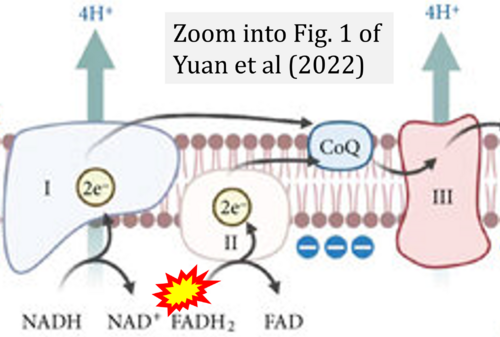
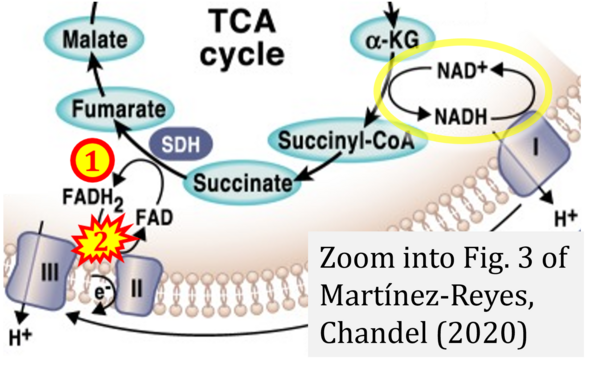
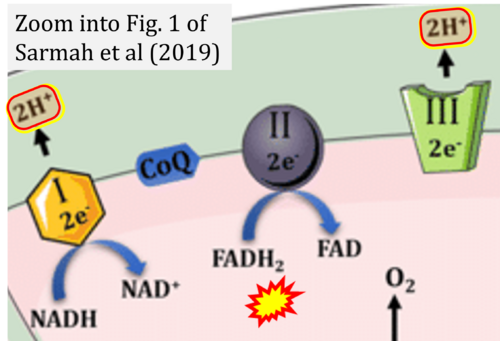
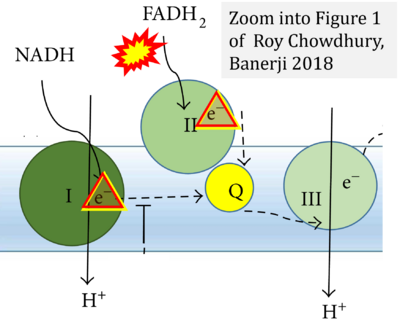
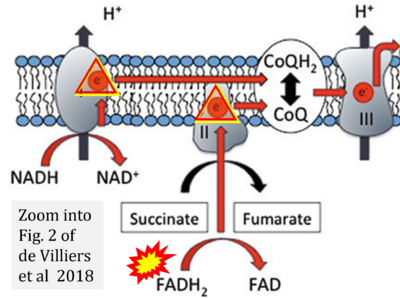
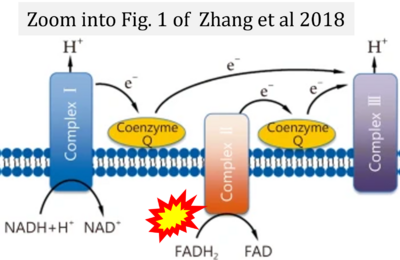
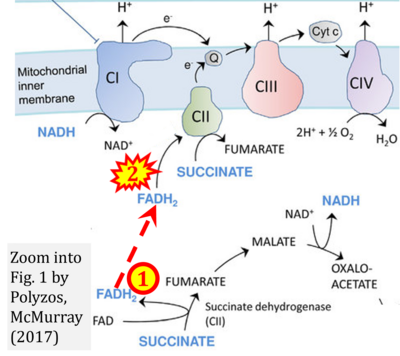
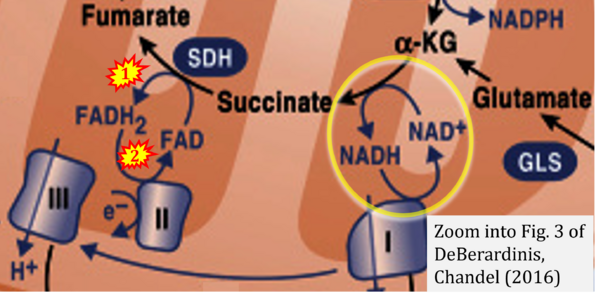
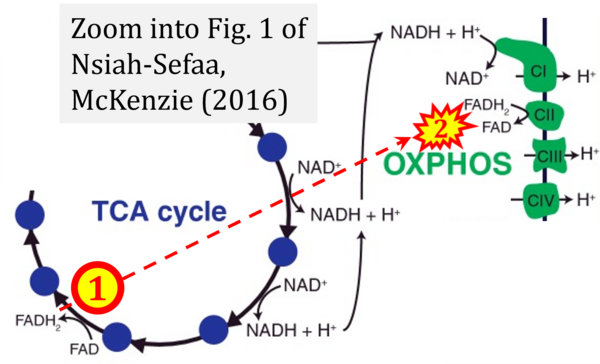
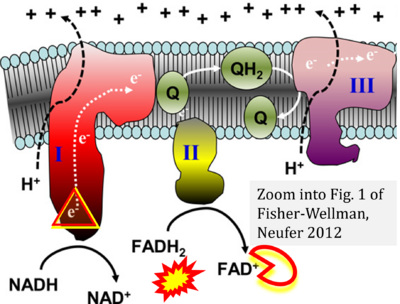
- (1) ambiguities in graphical representations: FADH2 is the product and substrate of CII in the same figure, e.g. DeBerardinis, Chandel (2016);
- (2) ambiguities with discrepancies between graphical representation and text: e.g. Figure 1 (error) and text in Fisher-Wellman, Neufer (2012) - 'Reducing equivalents (NADH, FADH2) provide electrons that flow through complex I, the ubiquinone cycle (Q/QH2), complex III, cytochrome c, complex IV, and to the final acceptor O2 to form water' (correct);
- (3) evolving errors in graphical representations: e.g. from Figure 6 (ambiguity) to Figure 1 (error) in Chandel (2021);
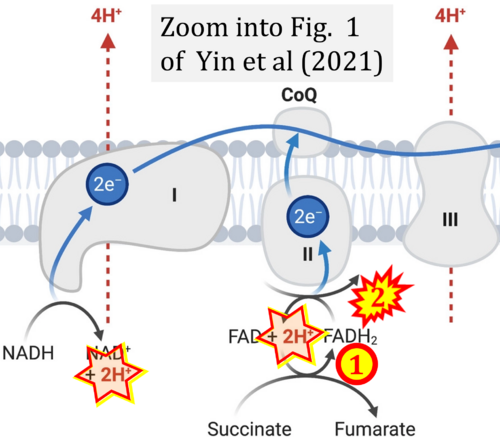
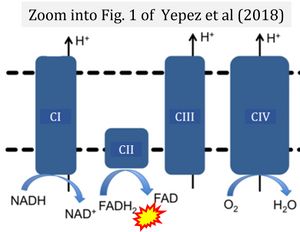
- (4) simple graphical errors: e.g. Yepez et al (2018), Zhang et al (2018); to
- (5) propagation of the error in the graphical representation solidified by text: e.g. Arnold, Finley (2022) with the following quotes:
- 'SDH reduces FAD to FADH2, which donates its electrons to complex II';
- 'each complete turn of the TCA cycle generates three NADH and one FADH2 molecules, which donate their electrons to complex I and complex II, respectively';
- 'complex I and complex II oxidize NADH and FADH2, respectively'.
- Ref. [1] Arnold PK, Finley LWS (2022) Regulation and function of the mammalian tricarboxylic acid cycle. J Biol Chem 299:102838. - »Bioblast link«
- Ref. [5] Chen CL, Zhang L, Jin Z, Kasumov T, Chen YR (2022) Mitochondrial redox regulation and myocardial ischemia-reperfusion injury. Am J Physiol Cell Physiol 322:C12-23. - »Bioblast link«
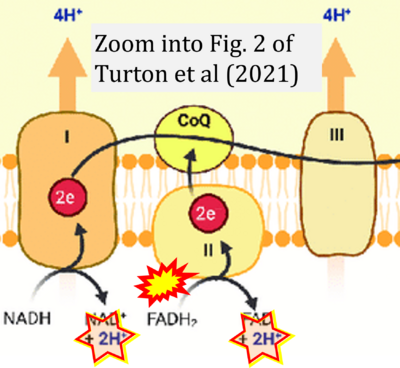
- Ref. [6] Turton N, Cufflin N, Dewsbury M, Fitzpatrick O, Islam R, Watler LL, McPartland C, Whitelaw S, Connor C, Morris C, Fang J, Gartland O, Holt L, Hargreaves IP (2022) The biochemical assessment of mitochondrial respiratory chain disorders. Int J Mol Sci 23:7487. - »Bioblast link«
- Ref. [7] Ahmad M, Wolberg A, Kahwaji CI (2022) Biochemistry, electron transport chain. StatPearls Publishing StatPearls [Internet]. Treasure Island (FL) - »Bioblast link«
- Ref. [8] Yuan Q, Zeng ZL, Yang S, Li A, Zu X, Liu J (2022) Mitochondrial stress in metabolic inflammation: modest benefits and full losses. Oxid Med Cell Longev 2022:8803404. - »Bioblast link«
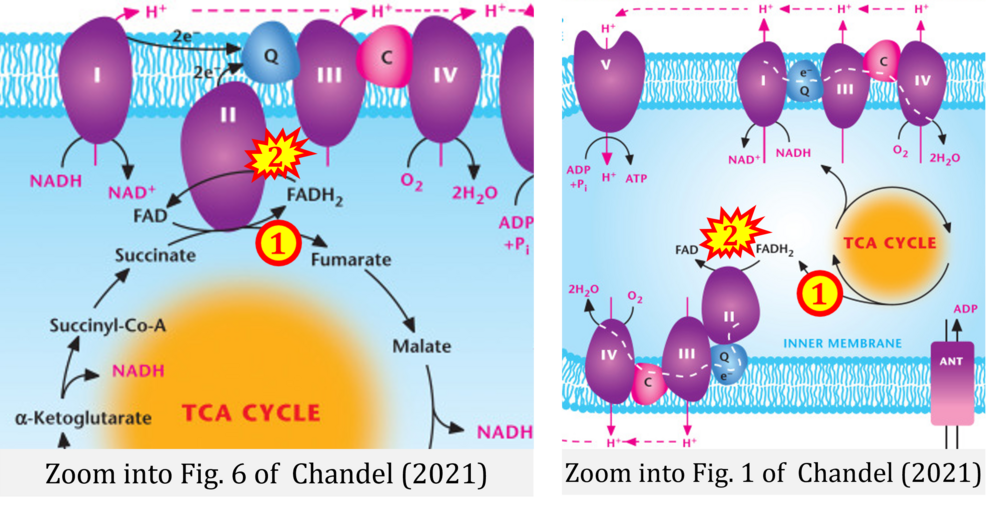
- Ref. [9] Chandel NS (2021) Mitochondria. Cold Spring Harb Perspect Biol 13:a040543. - »Bioblast link«
- Ref. [10] Yin M, O'Neill LAJ (2021) The role of the electron transport chain in immunity. FASEB J 35:e21974. - »Bioblast link«
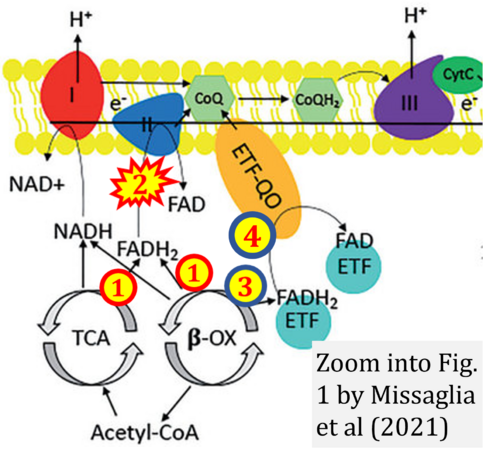
- Ref. [11] Missaglia S, Tavian D, Angelini C (2021) ETF dehydrogenase advances in molecular genetics and impact on treatment. Crit Rev Biochem Mol Biol 56:360-72. - »Bioblast link«
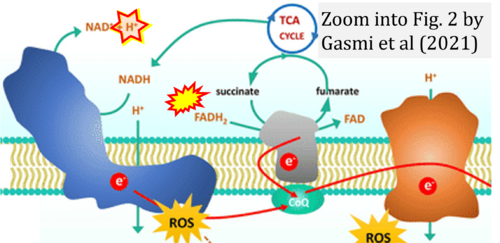
- Ref. [12] Gasmi A, Peana M, Arshad M, Butnariu M, Menzel A, Bjørklund G (2021) Krebs cycle: activators, inhibitors and their roles in the modulation of carcinogenesis. Arch Toxicol 95:1161-78. - »Bioblast link«
- Ref. [13] Turton N, Bowers N, Khajeh S, Hargreaves IP, Heaton RA (2021) Coenzyme Q10 and the exclusive club of diseases that show a limited response to treatment. Expert Opinion on Orphan Drugs 9:151-60. - »Bioblast link«
- Ref. [14] Martínez-Reyes I, Chandel NS (2020) Mitochondrial TCA cycle metabolites control physiology and disease. Nat Commun 11:102. - »Bioblast link«
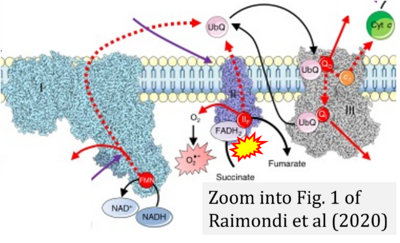
- Ref. [15] Raimondi V, Ciccarese F, Ciminale V (2020) Oncogenic pathways and the electron transport chain: a dangeROS liaison. Br J Cancer 122:168-81. - »Bioblast link«
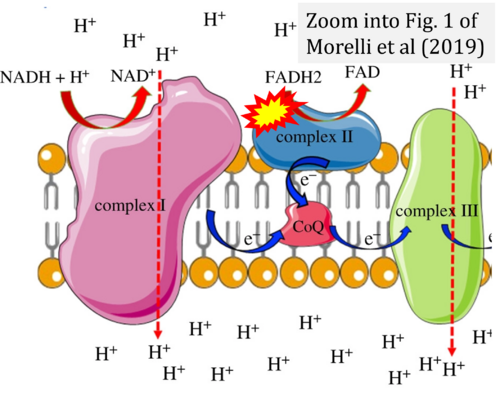
- Ref. [16] Morelli AM, Ravera S, Calzia D, Panfoli I (2019) An update of the chemiosmotic theory as suggested by possible proton currents inside the coupling membrane. Open Biol 9:180221. - »Bioblast link«
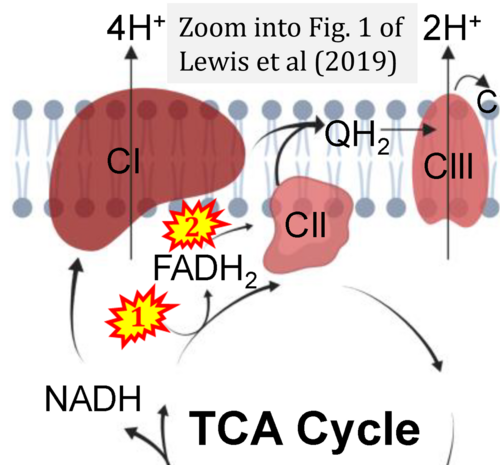
- Ref. [17] Lewis MT, Kasper JD, Bazil JN, Frisbee JC, Wiseman RW (2019) Quantification of mitochondrial oxidative phosphorylation in metabolic disease: application to Type 2 diabetes. Int J Mol Sci 20:5271. - »Bioblast link«
- Ref. [18] Sarmah D, Kaur H, Saraf J, Vats K, Pravalika K, Wanve M, Kalia K, Borah A, Kumar A, Wang X, Yavagal DR, Dave KR, Bhattacharya P (2019) Mitochondrial dysfunction in stroke: implications of stem cell therapy. Transl Stroke Res doi: 10.1007/s12975-018-0642-y - »Bioblast link«
- Ref. [19] Yépez VA, Kremer LS, Iuso A, Gusic M, Kopajtich R, Koňaříková E, Nadel A, Wachutka L, Prokisch H, Gagneur J (2018) OCR-Stats: Robust estimation and statistical testing of mitochondrial respiration activities using Seahorse XF Analyzer. PLOS ONE 13:e0199938. - »Bioblast link«
- Ref. [20] Roy Chowdhury S, Banerji V (2018) Targeting mitochondrial bioenergetics as a therapeutic strategy for chronic lymphocytic leukemia. Oxid Med Cell Longev 2018:2426712. - »Bioblast link«
- Ref. [21] de Villiers D, Potgieter M, Ambele MA, Adam L, Durandt C, Pepper MS (2018) The role of reactive oxygen species in adipogenic differentiation. Adv Exp Med Biol 1083:125-144. - »Bioblast link«
- Ref. [22] Zhang H, Feng YW, Yao YM (2018) Potential therapy strategy: targeting mitochondrial dysfunction in sepsis. Mil Med Res 5:41. - »Bioblast link«
- Ref. [23] Polyzos AA, McMurray CT (2017) The chicken or the egg: mitochondrial dysfunction as a cause or consequence of toxicity in Huntington's disease. Mech Ageing Dev 161:181-97. - »Bioblast link«
- Ref. [24] Jones PM, Bennett MJ (2017) Chapter 4 - Disorders of mitochondrial fatty acid β-oxidation. Elsevier In: Garg U, Smith LD , eds. Biomarkers in inborn errors of metabolism. Clinical aspects and laboratory determination:87-101. - »Bioblast link«
- Ref. [25] DeBerardinis RJ, Chandel NS (2016) Fundamentals of cancer metabolism. Sci Adv 2:e1600200. - »Bioblast link«
- Ref. [26] Nsiah-Sefaa A, McKenzie M (2016) Combined defects in oxidative phosphorylation and fatty acid β-oxidation in mitochondrial disease. Biosci Rep 36:e00313. - »Bioblast link«
- Ref. [27] Fisher-Wellman KH, Neufer PD (2012) Linking mitochondrial bioenergetics to insulin resistance via redox biology. Trends Endocrinol Metab 23:142-53. - »Bioblast link«
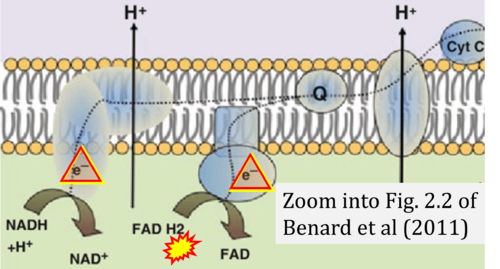
- Ref. [28] Benard G, Bellance N, Jose C, Rossignol R (2011) Relationships between mitochondrial dynamics and bioenergetics. In: Lu Bingwei (ed) Mitochondrial dynamics and neurodegeneration. Springer ISBN 978-94-007-1290-4:47-68. - »Bioblast link«
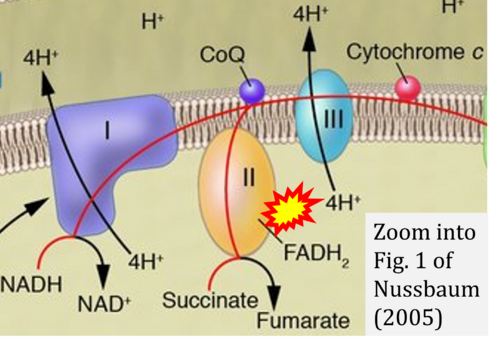
- Ref. [29] Nussbaum RL (2005) Mining yeast in silico unearths a golden nugget for mitochondrial biology. J Clin Invest 115:2689-91. - »Bioblast link«
- Ref. [30] Sanchez H, Zoll J, Bigard X, Veksler V, Mettauer B, Lampert E, Lonsdorfer J, Ventura-Clapier R (2001) Effect of cyclosporin A and its vehicle on cardiac and skeletal muscle mitochondria: relationship to efficacy of the respiratory chain. Br J Pharmacol 133:781-8. - »Bioblast link«
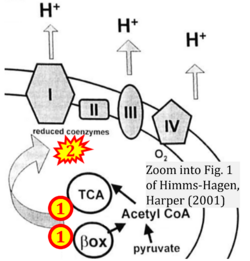
- Ref. [31] Himms-Hagen J, Harper ME (2001) Physiological role of UCP3 may be export of fatty acids from mitochondria when fatty acid oxidation predominates: an hypothesis. Exp Biol Med (Maywood) 226:78-84. - »Bioblast link«
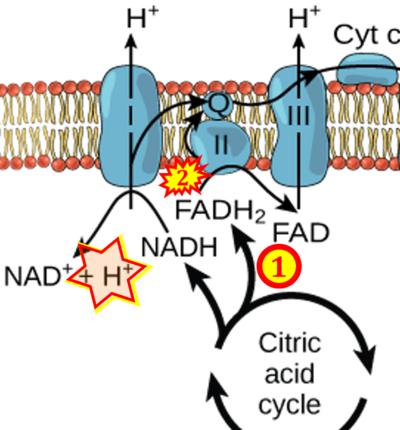
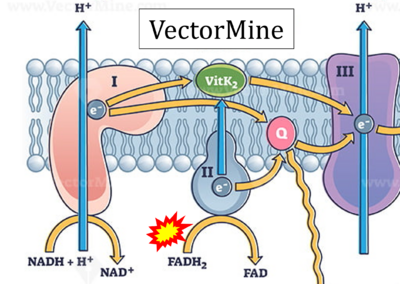
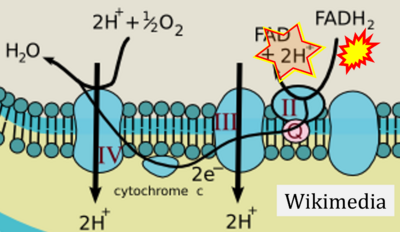
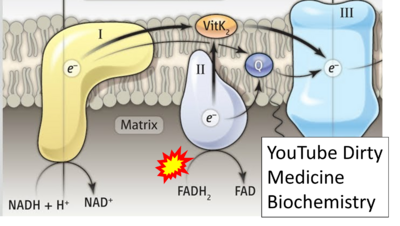
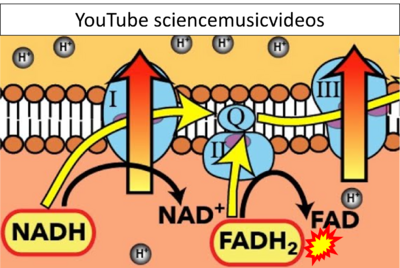
FADH2→CII misconceptions: Websites
- Website 1: Oxidative phosphorylation by OpenStax Biology (CC BY 3.0) got it wrong in figures and text, and the error is propagated further, with copies in (among several further links)
- Website 2: LibreTexts Biology
- Website 3: lumen Biology for Majors I
- Website 4: Concepts of Biology - 1st Canadian Edition by Charles Molnar and Jane Gair
- Website 5: Pharmaguideline
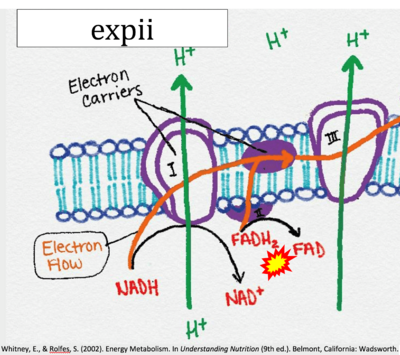
- Website 6: expii - By OpenStax College
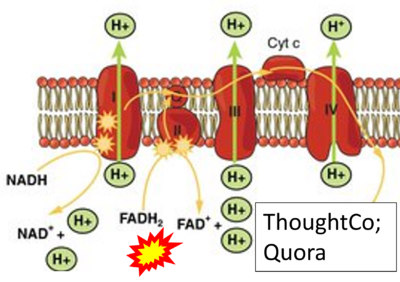
- Website 7: Quora
- Website 8: TeachMePhysiology.com
- Website 9: ThoughtCo
- Website 10: toppr.com
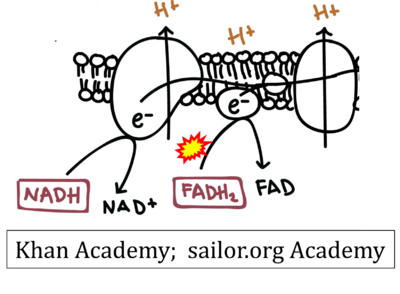
- Website 11: Khan Academy
- Website 12: saylor.org Academy
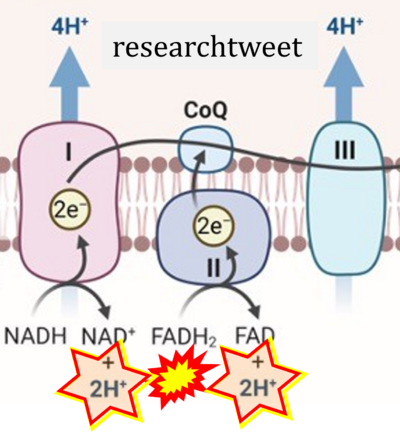
- Website 13: researchtweet
- Website 14: Microbe Notes
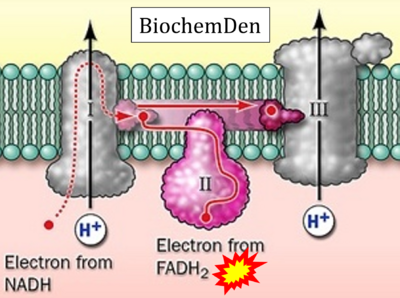
- Website 15: BiochemDen.com
- Website 16: biologydictionary.net
- Website 17: Conduct Science: "In Complex II, the enzyme succinate dehydrogenase in the inner mitochondrial membrane reduce FADH2 to FAD+. Simultaneously, succinate, an intermediate in the Krebs cycle, is oxidized to fumarate." - Comments: FAD does not have a postive charge. FADH2 is the reduced form, it is not reduced. And again: In CII, FAD is reduced to FADH2.
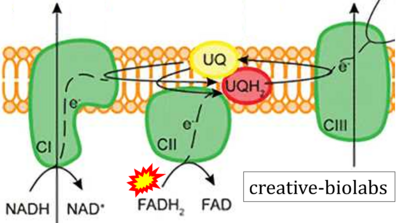
- Website 18: creative-biolabs.com
- Website 19: expii - Image source: By CNX OpenStax
- Website 20: Expii-Whitney, Rolfes 2002
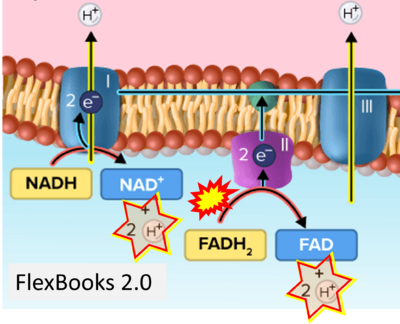
- Website 21: FlexBooks 2.0 > CK-12 Biology for High School
- Website 22: Hyperphysics
- Website 23: Jack Westin MCAT Courses
- Website 24: Labxchange Figure 8.15
- Website 25: nau.edu
- Website 26: ScienceDirect
- Website 27: ScienceFacts.no
- Website 28: SNC1D - BIOLOGY LESSON PLAN BLOG
- Website 29: unm.edu
- Website 30: VectorMine
- Website 31: Wikimedia
- Website 32: YouTube Dirty Medicine Biochemistry
- Website 33: YouTube sciencemusicvideos
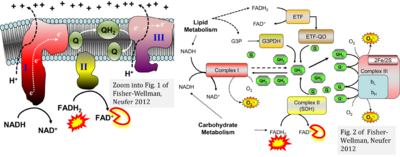
CII and fatty acid oxidation
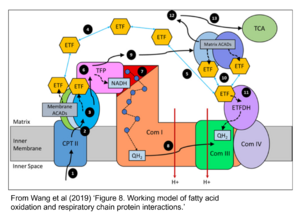
- Fatty acid oxidation requires electron transferring flavoprotein CETF and CI for electron entry into the Q-junction (Gnaiger 2020; Wang et al 2019; see figures on the right).
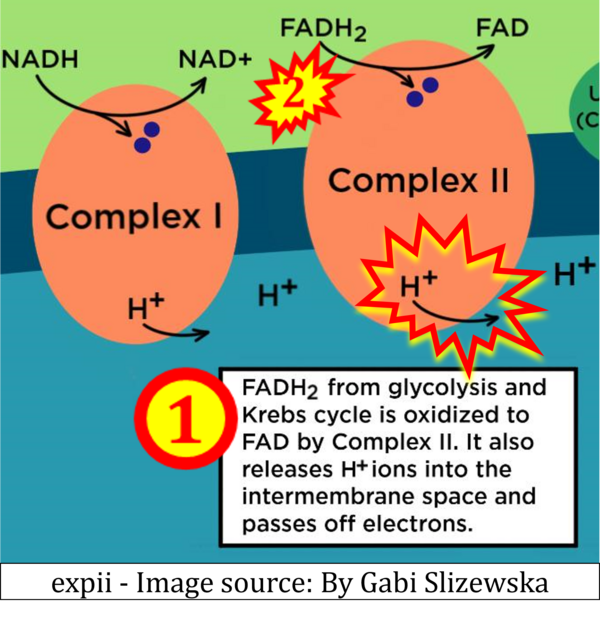
- Website 34: expii - Image source: By Gabi Slizewska
- "Since mitochondrial Complex II also participates in the oxidation of fatty acids (6), .." (quote from Lemmi et al 1990).
- Ref 6: Tzagoloff A (1982) Mitochondria. Plenum, New York.
- This quote is erroneous, since the textbook by Tzagoloff (1982) represents fatty acid oxidation in figures and text without any involvement of CII.
- Website 35: CHM333 LECTURES 37 & 38: 4/27 – 29/13 SPRING 2013 Professor Christine Hrycyna - Acyl-CoA dehydrogenase is listed under 'Electron transfer in Complex II'.
MitoPedia concepts: MiP concept
MitoPedia topics:
Enzyme,
Substrate and metabolite
Labels:
MitoPedia:FAT4BRAIN