Difference between revisions of "Gibbs energy"
From Bioblast
| (10 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
{{MitoPedia | {{MitoPedia | ||
|abbr=''G'' [J] | |abbr=''G'' [J] | ||
|description='''Gibbs energy''' ''G'' [J] is [[exergy]] which cannot be created internally (subscript i), but in contrast to [[internal-energy]] (d<sub>i</sub>''U''/d''t'' = 0) is not conserved but is dissipated (d<sub>i</sub>''G''/d''t'' < 0) in irreversible energy transformations. Exergy is available as [[work]] in reversible energy transformations, and can be partially conserved when the [[exergonic]] transformation is coupled to an [[endergonic]] transformation. | |description='''Gibbs energy''' ''G'' [J] is [[exergy]] which cannot be created internally (subscript i), but in contrast to [[internal-energy]] (d<sub>i</sub>''U''/d''t'' = 0) is not conserved but is dissipated (d<sub>i</sub>''G''/d''t'' < 0) in irreversible energy transformations at constant temperature and (barometric) pressure, ''T'',''p''. Exergy is available as [[work]] in reversible energy transformations (100 % [[efficiency]]), and can be partially conserved when the [[exergonic]] transformation is coupled to an [[endergonic]] transformation. | ||
|info=<u>[[Energy]]</u> | |info=<u>[[Energy]]</u> | ||
}} | }} | ||
[[File:Gibbs energy advancement.png|right|330px|link=Gnaiger_2020_BEC_MitoPathways#Chapter_8._Protonmotive_pressure_and_respiratory_control |Gibbs energy and advancement|thumb|Figure 8.5. Gibbs energy as a function of advancement of transformation in a closed isothermal system at constant pressure (modified from [[Gnaiger 2020 BEC MitoPathways]]).]] | [[File:Gibbs energy advancement.png|right|330px|link=Gnaiger_2020_BEC_MitoPathways#Chapter_8._Protonmotive_pressure_and_respiratory_control |Gibbs energy and advancement|thumb|Figure 8.5. Gibbs energy as a function of advancement of a transformation (0 = -1 A + 1 B) in a closed isothermal system at constant pressure, for ''μ''<sub>A</sub>° = ''μ''<sub>B</sub>° = 0 kJ·mol<sup>-1</sup> (modified from [[Gnaiger 2020 BEC MitoPathways]] - see Footnote 1).]] | ||
== Gibbs energy as a function of advancement == | == Gibbs energy as a function of advancement == | ||
Communicated by [[Gnaiger E]] 2022-10- | Communicated by [[Gnaiger E]] 2022-10-19 | ||
:::: In a transformation tr 0 = -1 A +1 B proceeding in a system with volume ''V'' at constant barometric pressure ''p'', the Gibbs energy of reactants A and B is | :::: In a transformation tr 0 = -1 A +1 B proceeding in a system with volume ''V'' at constant barometric pressure ''p'', the Gibbs energy of reactants A and B is | ||
| Line 12: | Line 13: | ||
<big>'''Eq. 1''': ''G'' = ''µ''<sub>A</sub>·''n''<sub>A</sub> + ''µ''<sub>B</sub>·''n''<sub>B</sub> [J] </big> | <big>'''Eq. 1''': ''G'' = ''µ''<sub>A</sub>·''n''<sub>A</sub> + ''µ''<sub>B</sub>·''n''<sub>B</sub> [J] </big> | ||
:::: A small change d''G'' at constant chemical potentials ''µ''<sub>''i''</sub> is due to a small advancement of | :::: A small change d<sub>tr</sub>''G'' at constant chemical potentials ''µ''<sub>''i''</sub> is due to a small advancement of a transformation tr, in closed or open isothermal systems, exchanging heat in equilibrium with an external thermostat at constant temperature, | ||
<big>'''Eq. 2''': d<sub>tr</sub>''G'' = ''µ''<sub>A</sub>·d<sub>tr</sub>''n''<sub>A</sub> + ''µ''<sub>B</sub>·d<sub>tr</sub>''n''<sub>B</sub> [J] </big> | <big>'''Eq. 2''': d<sub>tr</sub>''G'' = ''µ''<sub>A</sub>·d<sub>tr</sub>''n''<sub>A</sub> + ''µ''<sub>B</sub>·d<sub>tr</sub>''n''<sub>B</sub> [J] </big> | ||
:::: where the advancement d<sub>tr</sub>''ξ''<sub>''i''</sub> (''i'' = A or B) is | :::: where the [[advancement]] d<sub>tr</sub>''ξ''<sub>''i''</sub> (''i'' = A or B) is | ||
<big>'''Eq. 3''': d<sub>tr</sub>''ξ''<sub>''i''</sub> = d<sub>tr</sub>''n''<sub>A</sub>·''ν''<sub>A</sub><sup>-1</sup> = d<sub>tr</sub>''n''<sub>B</sub>·''ν''<sub>B</sub><sup>-1</sup> [mol] </big> | <big>'''Eq. 3''': d<sub>tr</sub>''ξ''<sub>''i''</sub> = d<sub>tr</sub>''n''<sub>A</sub>·''ν''<sub>A</sub><sup>-1</sup> = d<sub>tr</sub>''n''<sub>B</sub>·''ν''<sub>B</sub><sup>-1</sup> [mol] </big> | ||
:::: The isomorphic force of transformation Δ<sub>tr</sub>''F''<sub>''X''</sub> is the derivative of exergy per advancement, | :::: The total change of Gibbs energy d''G'' is the sum of all partial transformations, d''G'' = Σd<sub>tr<sub>''i''</sub></sub>''G'', where tr<sub>''i''</sub> = 1 to ''N'' transformation types — not to be confused with the internal Gibbs energy change d<sub>i</sub>''G'' due to [[Internal flow |internal transformations (i)]] only. | ||
:::: The isomorphic force of transformation Δ<sub>tr</sub>''F''<sub>''X''</sub> is the derivative of exergy per advancement (Gibbs force, compare [[affinity of reaction]]), | |||
<big>'''Eq. 4''': Δ<sub>tr</sub>''F''<sub>''X''</sub> = ∂''G''/∂<sub>tr</sub>''ξ''<sub>''X''</sub> [J·mol<sup>-1</sup>] </big> | <big>'''Eq. 4''': Δ<sub>tr</sub>''F''<sub>''X''</sub> = ∂''G''/∂<sub>tr</sub>''ξ''<sub>''X''</sub> [J·mol<sup>-1</sup>] </big> | ||
| Line 26: | Line 29: | ||
:::: Note that ∂''G'' ≝ d<sub>tr</sub>''G''. Then inserting Eq. 2 and Eq. 3 into Eq. 4, the force of transformation is expressed as | :::: Note that ∂''G'' ≝ d<sub>tr</sub>''G''. Then inserting Eq. 2 and Eq. 3 into Eq. 4, the force of transformation is expressed as | ||
<big>'''Eq. 5''': Δ<sub>tr</sub>''F''<sub>''X''</sub> = (''µ''<sub>A</sub>·d<sub>tr</sub>''n''<sub>A</sub> + ''µ''<sub>B</sub>·d<sub>tr</sub>''n''<sub>B</sub>)/ | <big>'''Eq. 5''': Δ<sub>tr</sub>''F''<sub>''X''</sub> = (''µ''<sub>A</sub>·d<sub>tr</sub>''n''<sub>A</sub> + ''µ''<sub>B</sub>·d<sub>tr</sub>''n''<sub>B</sub>)/d<sub>tr</sub>''ξ''<sub>''i''</sub> [J·mol<sup>-1</sup>] </big> | ||
:::: | :::: Using Eq. 3, Eq. 5 can be rewritten as | ||
<big>'''Eq. 6''': Δ<sub>tr</sub>''F''<sub>''X''</sub> = ''µ''<sub>A</sub>·d<sub>tr</sub>''n''<sub>A</sub>/(d<sub>tr</sub>''n''<sub>A</sub>·''ν''<sub>A</sub><sup>-1</sup>) + ''µ''<sub>B</sub>·d<sub>tr</sub>''n''<sub>B</sub>/(d<sub>tr</sub>''n''<sub>B</sub>·''ν''<sub>B</sub><sup>-1</sup>) [J·mol<sup>-1</sup>] </big> | <big>'''Eq. 6''': Δ<sub>tr</sub>''F''<sub>''X''</sub> = ''µ''<sub>A</sub>·d<sub>tr</sub>''n''<sub>A</sub>/(d<sub>tr</sub>''n''<sub>A</sub>·''ν''<sub>A</sub><sup>-1</sup>) + ''µ''<sub>B</sub>·d<sub>tr</sub>''n''<sub>B</sub>/(d<sub>tr</sub>''n''<sub>B</sub>·''ν''<sub>B</sub><sup>-1</sup>) [J·mol<sup>-1</sup>] </big> | ||
| Line 38: | Line 41: | ||
:::: In general, | :::: In general, | ||
<big>'''Eq. 8''': Δ<sub>tr</sub>''F''<sub>''X''</sub> = Σ''µ''<sub>''i''</sub>·''ν''<sub>''i''</sub> = Σ''F''tr<sub>''i''</sub>[J·mol<sup>-1</sup>] </big> | <big>'''Eq. 8''': Δ<sub>tr</sub>''F''<sub>''X''</sub> = Σ''µ''<sub>''i''</sub>·''ν''<sub>''i''</sub> = Σ''F''<sub>tr<sub>''i''</sub></sub> [J·mol<sup>-1</sup>] </big> | ||
:::: It may arouse curiosity, why the sign of difference Δ is used in the symbol, whereas the equation suggest a sum Σ in contrast to a difference. This is best explained by the fact that in various conventional contexts — such as the classical formulation of the ''pmF'' — the stoichiometric numbers (-1 and +1) are omitted, which yields | :::: It may arouse curiosity, why the sign of difference Δ is used in the symbol, whereas the equation suggest a sum Σ in contrast to a difference. This is best explained by the fact that in various conventional contexts — such as the classical formulation of the ''pmF'' — the stoichiometric numbers (-1 and +1) are omitted, which yields a difference Δ as an [[equivalence]], | ||
<big>'''Eq. 9''': Δ<sub>tr</sub>''F''<sub>''X''</sub> ≡ ''µ''<sub>B</sub> - ''µ''<sub>A</sub> [J·mol<sup>-1</sup>] </big> | <big>'''Eq. 9''': Δ<sub>tr</sub>''F''<sub>''X''</sub> ≡ ''µ''<sub>B</sub> - ''µ''<sub>A</sub> [J·mol<sup>-1</sup>] </big> | ||
:::: The conceptual importance of the [[stoichiometric number]]s | :::: The conceptual importance of the [[stoichiometric number]]s is emphasized by defining the term stoichiometric potential (Gnaiger 2020), analogous to combining d<sub>tr</sub>''n''<sub>A</sub>·''ν''<sub>A</sub><sup>-1</sup> in the expression of advancement (Eq. 3; see Eqs. 7 and 8), | ||
<big>'''Eq. 10''': ''F''<sub>tr''i''</sub> = ''µ''<sub>''i''</sub>·''ν''<sub>''i''</sub> [J·mol<sup>-1</sup>] </big> | <big>'''Eq. 10''': ''F''<sub>tr''i''</sub> = ''µ''<sub>''i''</sub>·''ν''<sub>''i''</sub> [J·mol<sup>-1</sup>] </big> | ||
:::: To get acquainted with the meaning of subscripts such as 'tr' used above, consult »[[Iconic_symbols#Abbreviation_of_iconic_symbols |Abbreviation of iconic symbols]]. | |||
'''References''' | '''References''' | ||
::::* Gnaiger E (2020) Mitochondrial pathways and respiratory control. An introduction to OXPHOS analysis. 5th ed. Bioenerg Commun 2020.2. https://doi.org/10.26124/bec:2020-0002 | ::::* Gnaiger E (2020) Mitochondrial pathways and respiratory control. An introduction to OXPHOS analysis. 5th ed. Bioenerg Commun 2020.2. https://doi.org/10.26124/bec:2020-0002 | ||
---- | |||
'''Footnote 1''' | |||
:::: The original Figure 8.5 shows ∂<sub>tr</sub>''ξ''<sub>''X''</sub> = ∂<sub>tr</sub>''n''<sub>''X''</sub>∙''ν''<sub>''X''</sub><sup>-1</sup> instead of d<sub>tr</sub>''ξ''<sub>''X''</sub> = d<sub>tr</sub>''n''<sub>''X''</sub>∙''ν''<sub>''X''</sub><sup>-1</sup>. The formal inconsistency was pointed out by [[Kuntic Marin |Marin Kuntic]] during the [[MiPNet27.05_Schroecken_BEC_tutorial-Living_Communications_pmP |BEC tutorial-Living Communications: pmF to pmP (Schroecken 2022 Sep 30-Oct 03)]]. | |||
{{Keywords: Energy and exergy}} | {{Keywords: Energy and exergy}} | ||
Latest revision as of 06:46, 9 February 2024
Description
Gibbs energy G [J] is exergy which cannot be created internally (subscript i), but in contrast to internal-energy (diU/dt = 0) is not conserved but is dissipated (diG/dt < 0) in irreversible energy transformations at constant temperature and (barometric) pressure, T,p. Exergy is available as work in reversible energy transformations (100 % efficiency), and can be partially conserved when the exergonic transformation is coupled to an endergonic transformation.
Abbreviation: G [J]
Reference: Energy
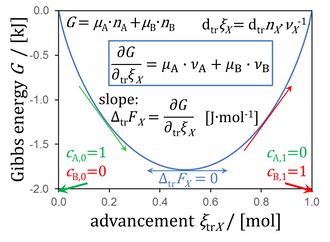
Figure 8.5. Gibbs energy as a function of advancement of a transformation (0 = -1 A + 1 B) in a closed isothermal system at constant pressure, for μA° = μB° = 0 kJ·mol-1 (modified from Gnaiger 2020 BEC MitoPathways - see Footnote 1).
Gibbs energy as a function of advancement
Communicated by Gnaiger E 2022-10-19
- In a transformation tr 0 = -1 A +1 B proceeding in a system with volume V at constant barometric pressure p, the Gibbs energy of reactants A and B is
Eq. 1: G = µA·nA + µB·nB [J]
- A small change dtrG at constant chemical potentials µi is due to a small advancement of a transformation tr, in closed or open isothermal systems, exchanging heat in equilibrium with an external thermostat at constant temperature,
Eq. 2: dtrG = µA·dtrnA + µB·dtrnB [J]
- where the advancement dtrξi (i = A or B) is
Eq. 3: dtrξi = dtrnA·νA-1 = dtrnB·νB-1 [mol]
- The total change of Gibbs energy dG is the sum of all partial transformations, dG = ΣdtriG, where tri = 1 to N transformation types — not to be confused with the internal Gibbs energy change diG due to internal transformations (i) only.
- The isomorphic force of transformation ΔtrFX is the derivative of exergy per advancement (Gibbs force, compare affinity of reaction),
Eq. 4: ΔtrFX = ∂G/∂trξX [J·mol-1]
- Note that ∂G ≝ dtrG. Then inserting Eq. 2 and Eq. 3 into Eq. 4, the force of transformation is expressed as
Eq. 5: ΔtrFX = (µA·dtrnA + µB·dtrnB)/dtrξi [J·mol-1]
- Using Eq. 3, Eq. 5 can be rewritten as
Eq. 6: ΔtrFX = µA·dtrnA/(dtrnA·νA-1) + µB·dtrnB/(dtrnB·νB-1) [J·mol-1]
- This yields the force as the sum of stoichiometric potentials, summarized in Figure 8.5 (Chapter 8; Gnaiger 2020 BEC MitoPathways),
Eq. 7: ΔtrFX = µA·νA + µB·νB [J·mol-1]
- In general,
Eq. 8: ΔtrFX = Σµi·νi = ΣFtri [J·mol-1]
- It may arouse curiosity, why the sign of difference Δ is used in the symbol, whereas the equation suggest a sum Σ in contrast to a difference. This is best explained by the fact that in various conventional contexts — such as the classical formulation of the pmF — the stoichiometric numbers (-1 and +1) are omitted, which yields a difference Δ as an equivalence,
Eq. 9: ΔtrFX ≡ µB - µA [J·mol-1]
- The conceptual importance of the stoichiometric numbers is emphasized by defining the term stoichiometric potential (Gnaiger 2020), analogous to combining dtrnA·νA-1 in the expression of advancement (Eq. 3; see Eqs. 7 and 8),
Eq. 10: Ftri = µi·νi [J·mol-1]
- To get acquainted with the meaning of subscripts such as 'tr' used above, consult »Abbreviation of iconic symbols.
References
- Gnaiger E (2020) Mitochondrial pathways and respiratory control. An introduction to OXPHOS analysis. 5th ed. Bioenerg Commun 2020.2. https://doi.org/10.26124/bec:2020-0002
Footnote 1
- The original Figure 8.5 shows ∂trξX = ∂trnX∙νX-1 instead of dtrξX = dtrnX∙νX-1. The formal inconsistency was pointed out by Marin Kuntic during the BEC tutorial-Living Communications: pmF to pmP (Schroecken 2022 Sep 30-Oct 03).
- »Bioblast links: Energy and exergy - >>>>>>> - Click on [Expand] or [Collapse] - >>>>>>>
- Units
- Joule [J]; 1 J = 1 N·m = 1 V·C; 1 cal = 4.184 J
- Units
- Fundamental relationships
- » Energy
- » Exergy
- » Extensive quantity
- Fundamental relationships
- Contrast
- » Force
- » Pressure
- » Intensive quantity
- Contrast
- Forms of energy
- » Internal-energy dU
- » Enthalpy dH
- » Heat deQ
- » Bound energy dB
- Forms of energy
- Forms of exergy
- » Helmholtz energy dA
- » Gibbs energy dG
- » Work deW
- » Dissipated energy diD
- Forms of exergy
MitoPedia concepts:
Ergodynamics
