Semantic search
| Term | Abbreviation | Description |
|---|---|---|
| Carnitine palmitoyltransferase II | CPT-II | Carnitine palmitoyltransferase II (CPT-II, also known as carnitine acyltransferase II) is part of the carnitine shuttle which is responsible for the mitochondrial transport of long-chain fatty acids. CPT-II is located on the inner side of the mtIM and converts the acylcarnitines (produced in the reaction catalyzed by carnitine palmitoyltransferase I) to carnitine and acyl-CoAs, which undergo ß-oxidation in the mitochondrial matrix. Free carnitines are transported out of the mitochondrial matrix in exchange for acyl-carnitines via an integral mtIM protein carnitine-acylcarnitine translocase (CACT). Short- and medium-chain fatty acids do not require the carnitine shuttle for mitochondrial transport. |
| Carnitine-acylcarnitine translocase | CACT | Carnitine-acylcarnitine translocase (CACT) is part of the carnitine shuttle which mediates the mitochondrial transport of long-chain fatty acids where the fatty acid oxidation occurs. CACT is an internal mt-IM protein and transports acylcarnitines into the mitochondrial matrix in exchange for free carnitine. |
| Carrier control titrations | Most of the nonpolar compounds have to be diluted in organic solvents such as DMSO or acetonitrile in order to use them for the titrations in the SUIT protocols. However, the solvent (carrier) itself could affect the mitochondrial physiology and promote alterations that we need to take into account. For this reason, it is necessary to run in parallel to our treatment experiment a control experiment on which we will add a carrier control titration to test if it affects our sample or not. | |
| Catalase | Ctl | Catalase catalyzes the dismutation of hydrogen peroxide to water and oxygen. Perhaps all cells have catalase, but mitochondria of most cells lack catalase. Cardiac mitochondria are exceptional in having mt-catalase activity (rat heart mitochondria: Radi et al 1991; mouse heart mitochondria: Rindler et al 2013). Hydroxylamine is an inhibitor of catalase, which is also inhibited by cyanide and azide. Mitochondrial respiration medium MiR05 was developed considering the intracellular conditions of mitochondria in living cells. In mitochondrial preparations, enzymes and substrates present in the cytosol (such as catalase) are diluted when the plasma membrane is removed. Therefore, the addition of catalase is recommended when working with mitochondrial preparations, to consume any H2O2 generated during the assay. |
| Catalytic activity | kat | Catalytic activity of an enzyme is measured by an enzyme assay and is expressed in units of katal (kat [mol∙s-1]). More commonly (but not conforming to SI units or IUPAC recommendations) enzyme activity is expressed in units U [mol∙min-1]. |
| Cataplerosis | Cataplerosis is the exit of TCA cycle intermediates from the mt-matrix space. | |
| Categories of SUIT protocols | SUIT-catg |
Categories of SUIT protocols group SUIT protocols according to all substrate types involved in a protocol (F, N, S, Gp), independent of the sequence of titrations of substrates and inhibitors which define the Electron-transfer-pathway states. The N-type substrates are listed in parentheses, independent of the sequence of titrations. ROX states may or may not be included in a SUIT protocol, which does not change its category. Similarly, the CIV assay may or may not be added at the end of a SUIT protocol, without effect on the category of a SUIT protocol.
|
| Cell Symposia |  Organized by the editors of Cell Press's leading journals, Cell Symposia bring together exceptional speakers and scientists to discuss topics at the forefront of scientific research. Organized by the editors of Cell Press's leading journals, Cell Symposia bring together exceptional speakers and scientists to discuss topics at the forefront of scientific research. | |
| Cell count and normalization in HRR | Nce | The cell count Nce is the number of cells, expressed in the abstract unit [x] (1 Mx = 106 x). The elementary entity cell Uce [x] is the real unit, the 'single individual cell'. A cell count is the multitude or number N of cells, Nce = N·Uce (Gnaiger MitoFit Preprints 2020.4). Normalization of respiratory rate by cell count yields oxygen flow IO2 expressed in units [amol·s-1·x-1] (=10-18 mol·s-1·x-1). |
| Cell culture media | Cell culture media, like RPMI or DMEM, used for HRR of living cells. | |
| Cell respiration | Cell respiration channels metabolic fuels into the chemiosmotic coupling (bioenergetic) machinery of oxidative phosphorylation, being regulated by and regulating oxygen consumption (or consumption of an alternative final electron acceptor) and molecular redox states, ion gradients, mitochondrial (or microbial) membrane potential, the phosphorylation state of the ATP system, and heat dissipation in response to intrinsic and extrinsic energy demands. See also respirometry. In internal or cell respiration in contrast to fermentation, redox balance is maintained by external electron acceptors, transported into the cell from the environment. The chemical potential between electron donors and electron acceptors drives the electron transfer pathway, generating a chemiosmotic potential that in turn drives ATP synthesis. | |
| Cellular substrates | Ce; Cm | (1) Cellular substrates in vivo, endogenous; Ce. (2) Cellular substrates in vivo, with exogenous substrate supply from culture medium or serum; Cm.
|
| Chamber volume | The chamber volume of the O2k is 2.0 mL or 0.5 mL of aqueous medium with or without sample, excluding the volume of the stirrer and the volume of the capillary of the stopper (see: Cell count and normalization in HRR). A modular extension of the O2k, the O2k-sV-Module, was specifically developed to perform high-resolution respirometry with reduced amounts of biological sample, and all components necessary for the smaller operation volume of 0.5 mL. | |
| Channel | F7 | » See O2k signals and output |
| Charge | Qel [C] | Charge Qel is the quantity of electricity expressed in the SI unit coulomb [C]. QelX [C] indicates the charge carried by the quantity of a specified ion X. |
| Charge number | zX | The charge number of an ion X or electrochemical reaction with unit stoichiometric number of X is the particle charge [C·x-1] divided by the elementary charge [C·x-1]. The particle charge QNX is the charge per count of ions X or per ion X transferred in the reaction as defined in the reaction equation. |
| Check for updates - DatLab | Check for updates: Frequently check for updated DatLab 8 versions and follow the simple installation instruction if your computer running DatLab (Linux or Windows) is connected to internet. Alternatively, use a different computer connected to internet, download the update for Linux, and transfer it to the computer operating DatLab by USB (e.g. the O2k integrated PC). More information: Oroboros Marketplace - DatLab | |
| Chemical background | CHB, Chb |  Chemical background Chb is due to autooxidation of the reagents. During CIV assays, ascorbate and TMPD are added to maintain cytochrome c in a reduced state. External cytochrome c may be included in the CIV assay. The autooxidation of these compounds is linearly oxygen-dependent down to approximately 50 µM oxygen and responsible for the chemical background oxygen flux after the inhibition of CIV. Oxygen flux due to the chemical reaction of autooxidation must be corrected for the instrumental O2 background. The correction for chemical background is necessary to determine CIV activity, in which case the instrumental O2 background and chemical background may be combined in an overall correction term. Chemical background Chb is due to autooxidation of the reagents. During CIV assays, ascorbate and TMPD are added to maintain cytochrome c in a reduced state. External cytochrome c may be included in the CIV assay. The autooxidation of these compounds is linearly oxygen-dependent down to approximately 50 µM oxygen and responsible for the chemical background oxygen flux after the inhibition of CIV. Oxygen flux due to the chemical reaction of autooxidation must be corrected for the instrumental O2 background. The correction for chemical background is necessary to determine CIV activity, in which case the instrumental O2 background and chemical background may be combined in an overall correction term. |
| Chemical potential | µB [J/mol] | The chemical potential of a substance B, µB [J/mol], is the partial derivative of Gibbs energy, G [J], per amount of B, nB [mol], at constant temperature, pressure, and composition other than that of B, µB = (∂G/∂nB)T,p,nj≠B The chemical potential of a solute in solution is the sum of the standard chemical potential under defined standard conditions and a concentration (activity)-dependent term, µB = µB° + RT ln(aB) The standard state for the solute is refered to ideal behaviour at standard concentration, c° = 1 mol/L, exhibiting infinitely diluted solution behaviour [1]. µB° equals the standard molar Gibbs energy of formation, ΔfGB° [kJ·mol-1]. The formation process of B is the transformation of the pure constituent elements to one mole of substance B, with all substances in their standard state (the most stable form of the element at 100 kPa (1 bar) at the specified temperature) [2]. |
| Chinese Society of Mitochondrial Research and Medicine | Chinese-Mit | The Chinese Society of Mitochondrial Research and Medicine (Chinese-Mit) is a member of ASMRM. |
| Chinese numerals | Chinese numerals The Arabic numeral system used today in China was introduced to China by the Europeans in the early 17th century. But the Chinese character-based number systems are still in use. The financial numerals are used only when writing an amount on a form for remitting money at a bank. They function as anti-fraud numerals. The character 零 (zero) appeared very early in ancient Chinese writing. However, at that time, it did not mean "nothing", but "bits and pieces", "not much". 一百零五(105) means in Chinese: In addition to a hundred, there is a fraction of five. With the introduction of the Arabic numerals, 105 is exactly pronounced “one hundred zero five”, the character 零 corresponds exactly to the symbol 0. Thus, the character 零has the meaning of 0. But the character 〇 was one of the Chinese characters created and promulgated by the only empress (with greater achievements than countless emperors) in the history of China in 690 AD (much later than the invention of 0 in India) for the purpose of demonstrating her power. At that time the character 〇 meant “star”, representing a round planet. It is now used as a synonym for the 零 (zero). | |
| Chloroplasts | pt? | Chloroplasts (Greek chloros: green; plastes: the one who forms) are small structures within the cells that conduct photosynthesis. They are a type of organelle called plastids that are present in eukaryotic plant cells (algae, aquatic and terrestrial plants) and characterized by having two membranes and a high concentration of the pigment Chlorophyll. Like mitochondria, they originated through the endosymbiosis of a cyanobacteria by an early eukaryotic cell and they have their own DNA which replicates during cell division. In addition to photosynthesis, in their internal matrix called stroma they also carry out other metabolic functions within the plant cells such as fatty acid synthesis or amino acid synthesis. |
| Chlororespiration | In chlororespiration oxygen is consumed by a putative respiratory electron transfer system (ETS) within the thylakoid membrane of the chloroplasts and ATP is produced. It is a process that involves the interaction with the photosynthetic ETS in which NAD(P)H dehydrogenase transfers electrons to oxygen with the assistance of the photosynthetic plastoquinone (PQ), which acts as a non-photochemical redox carrier. Initially described in the unicellular alga Chlamydomonas reindhartdii, chlororespiration was highly disputed for years until the discovery of a NAD(P)H-dehydrogenase (NDH) complex (plastidic encoded) and plastid terminal oxidase (PTOX) (nuclear encoded) in higher-plant chloroplasts. PTOX is homologous to the plant mitochondrial alternative oxidase and has the role of preventing the over-reduction of the PQ pool while the NDH complexes provide a gateway for the electrons to form the ETS and consume oxygen. As a result of this process there is a cyclic electron flow around Photosystem I (PSI) that is activated under stress conditions acting as a photoprotection mechanism and could be involved in protecting against oxidative stress. | |
| Choline dehydrogenase | Choline dehydrogenase (EC 1.1.99.1) is bound to the inner mt-membrane, oxidizes choline in kidney and liver mitochondria, with electron transfer into the Q-junction, and is thus part of the Electron transfer pathway. Analogous to succinate dehydrogenase (CII), electron transfer from choline dehydrogenase is FAD-linked downstream to Q. Choline is an ET-pathway substrate types 3. | |
| Citrate | citrate, C6H5O7-3, is a tricarboxylic acid trianion, intermediate of the TCA cycle, obtained by deprotonation of the three carboxy groups of citric acid. Citrate is formed from oxaloacetate and acetyl-CoA through the catalytic activity of the citrate synthase. In the TCA cycle, citrate forms isocitrate by the activity of the aconitase. Citrate can be transported out of the mitochondria by the tricarboxylate transport, situated in the inner mitochondrial membrane. The transport occurs as an antiport of malate from the cytosol and it is a key process for fatty acid and oxaloacetate synthesis in the cytosol. | |
| Citrate synthase | CS | Condensation of oxaloacetate with acetyl-CoA yields citrate as an entry into the TCA cycle. CS is located in the mt-matrix. CS activity is frequently used as a functional marker of the amount of mitochondria (mitochondrial elementary marker, mtE) for normalization of respiratory flux. |
| Citreoviridin | Citreoviridin is an inhibitor of the ATP synthase which, differently from the FO subunit binding inhibitor oligmycin, binds to the F1 subunit of the ATP synthase. | |
| Close and delete file - DatLab | Close and delete a file. | |
| Closed chamber | C | The O2k-chamber can be used as a closed system or open system. Gas bubbles must be avoided. |
| Closed system | A closed system is a system with boundaries that allow external exchange of energy (heat and work), but do not allow exchange of matter. A limiting case is light and electrons which cross the system boundary when work is exchanged in the form of light or electric energy. If the surroundings are maintained at constant temperature, and heat exchange is rapid to prevent the generation of thermal gradients, then the closed system is isothermal. A frequently considered case are closed isothermal systems at constant pressure (and constant volume with aqueous solutions). Changes of closed systems can be partitioned according to internal and external sources. Closed systems may be homogenous (well mixed and isothermal), continuous with gradients, or discontinuous with compartments (heterogenous). | |
| Coenzyme | A coenzyme or cosubstrate is a cofactor that is attached loosely and transiently to an enzyme, in contrast to a prosthetic group that is attached permanently and tightly. The coenzyme is required by the corresponding enzyme for its activity (IUPAC definition). A coenzyme is 'a low-molecular-weight, non-protein organic compound participating in enzymatic reactions as dissociable acceptor or donor of chemical groups or electrons' (IUPAC definition). | |
| Coenzyme A | CoA | Coenzyme A is a coenzyme playing an essential role in the tricarboxylic acid cycle (oxidation of pyruvate to acetyl-CoA) and fatty acid oxidation. CoA is a thiol that reacts with carboxylic acids to form CoA-activated thioesters. |
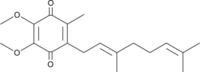
| Coenzyme Q | Q, CoQ | Coenzyme Q or ubiquinone (2,3-dimethoxy-5-methyl-6-polyprenyl-1,4-benzoquinone) was discovered in 1957 by the group of Crane. It is a lipid composed of a benzoquinone ring with an isoprenoid side chain, two methoxy groups and one methyl group. The length of the isoprenoid chain varies depending on the species; for example, six isoprenoid units (CoQ6) is the most commonly found CoQ in Saccharomyces cerevisiae, eight units in Escherichia coli (CoQ8), nine units in Caenorhabditis elegans and rodents (CoQ9), ten units in humans (CoQ10), and some species have more than one CoQ form, e.g. human and rodent mitochondria contain different proportions of CoQ9 and CoQ10. These redox compounds exist in three different forms: quinone (oxidized), quinol (reduced), and an intermediate semiquinone. More details » Q-junction |
| Coenzyme Q2 | CoQ2 | Coenzyme Q2 or ubiquinone-2 (CoQ2) is a quinone derivate composed of a benzoquinone ring with an isoprenoid side chain consisting of two isoprenoid groups, with two methoxy groups, and with one methyl group. In HRR it is used as a Q-mimetic to detect the redox changes of coenzyme Q at the Q-junction in conjunction with the Q-Module, since the naturally occurring long-chain coenzyme Q (e.g. CoQ10) is trapped within membrane boundaries. CoQ2 can react both with mitochondrial complexes (e.g. CI, CII and CIII) at their quinone-binding sites and with the detecting electrode. |
| Cofactor | A cofactor is 'an organic molecule or ion (usually a metal ion) that is required by an enzyme for its activity. It may be attached either loosely (coenzyme) or tightly (prosthetic group)' (IUPAC definition). | |
| Cole 2000 BMJ | ||
| Comma for separating a term and its abbreviation | , | Should we used a comma for separating a term and its abbreviation in the text? The SI Brochure frequently does not use a comma. The comma might be added, if it helps to clarify the distinction between the term and its abbreviation. The example “reduced Q fraction, Qr” – the sequence of Q and Qr may be confusing without comma. There will always be examples, where it is not clear, if a comma is needed. |
| Communication - mitochondria and the patient | Mitochondria and the patient: communication between patients, medical professionals, scientists, and the public | |
| Comorbidity | Comorbidities are common in obesogenic lifestyle-induced early aging. These are preventable, non-communicable diseases with strong associations to obesity. In many studies, cause and effect in the sequence of onset of comorbidities remain elusive. Chronic degenerative diseases are commonly obesity-induced. The search for the link between obesity and the etiology of diverse preventable diseases lead to the hypothesis, that mitochondrial dysfunction is the common mechanism, summarized in the term 'mitObesity'. | |
| Company of Scientists | CompaSci |
The Company of Scientists evolves as a concept for implementing scientific innovations on the market. |
| Comparison of respirometric methods | The comparison of respirometric methods provides the basis to evaluate different instrumental platforms and different mitochondrial preparations, as a guide to select the best approach and to critically evaluate published results. | |
| Complex I | CI | Complex I, NADH:ubiquinone oxidoreductase (EC 1.6.5.3), is an enzyme complex of the Electron transfer pathway, a proton pump across the inner mt-membrane, responsible for electron transfer to ubiquinone from NADH formed in the mt-matrix. CI forms a supercomplex with Complex III. There is a widespread ambiguity on the 'lonely H+ (the lonely hydron)' surrounding Complex I: CI ambiguities. |
| Complex I&II-linked substrate state | NS | See NS-pathway control state (previous: CI&II-linked) |
| Complex I-linked substrate state | N | See N-pathway control state (previous: CI-linked) versus Complex I |
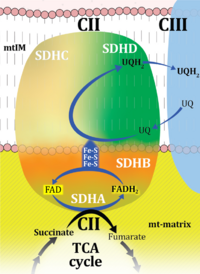
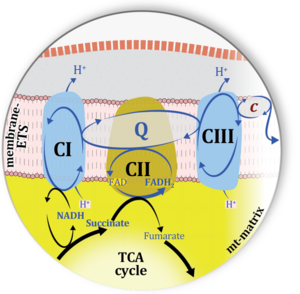
| Complex II | CII |
Complex II or succinate:quinone oxidoreductase (SQR) is the only membrane-bound enzyme in the TCA cycle and is part of the electron transfer pathway. The reversible oxidoreduction of succinate and fumarate is catalyzed in a soluble domain and coupled to the reversible oxidoreduction of quinol and quinone in the mitochondrial inner membrane. CII consists in most species of four subunits. The flavoprotein succinate dehydrogenase is the largest polypeptide of CII, located on the matrix face of the mt-inner membrane. Succinate:quinone oxidoreductases (SQRs, SDHABCD) favour oxidation of succinate and reduction of quinone in the canonical forward direction of the TCA cycle and electron transfer into the Q-junction. In contrast, quinol:fumarate reductases (QFRs, fumarate reductases, FRDABCD) tend to operate in the reverse direction reducing fumarate and oxidizing quinol. |
| Complex II ambiguities | CII ambiguities | The current narrative that the reduced coenzymes NADH and FADH2 feed electrons from the tricarboxylic acid (TCA) cycle into the mitochondrial electron transfer system can create ambiguities around respiratory Complex CII. Succinate dehydrogenase or CII reduces FAD to FADH2 in the canonical forward TCA cycle. However, some graphical representations of the membrane-bound electron transfer system (ETS) depict CII as the site of oxidation of FADH2. This leads to the false believe that FADH2 generated by electron transferring flavoprotein (CETF) in fatty acid oxidation and mitochondrial glycerophosphate dehydrogenase (CGpDH) feeds electrons into the ETS through CII. In reality, NADH and succinate produced in the TCA cycle are the substrates of Complexes CI and CII, respectively, and the reduced flavin groups FMNH2 and FADH2 are downstream products of CI and CII, respectively, carrying electrons from CI and CII into the Q-junction. Similarly, CETF and CGpDH feed electrons into the Q-junction but not through CII. The ambiguities surrounding Complex II in the literature call for quality control, to secure scientific standards in current communications on bioenergetics and support adequate clinical applications. |
| Complex II-linked substrate state | SRot, S | See S-pathway control state (previous: CII-linked) |
| Complex III | CIII | Complex III or coenzyme Q : cytochrome c - oxidoreductase, sometimes also called the cytochrome bc1 complex is a complex of the electron transfer pathway. It catalyzes the reduction of cytochrome c by oxidation of coenzyme Q (CoQ) and the concomitant pumping of 4 protons from the cathodic (negative) mitochondrial matrix to the anodic (positive) intermembrane space. |
| Complex IV | CIV | Complex IV or cytochrome c oxidase is the terminal oxidase of the mitochondrial electron transfer system, reducing oxygen to water, with reduced cytochrome c as a substrate. Concomitantly to that, CIV pumps protons against the electrochemical protonmotive force. CIV is frequently abbreviated as COX or CcO. It is the 'ferment' (Atmungsferment) of Otto Warburg, shown to be related to the cytochromes discovered by David Keilin. |
| Concentration | c [mol·L-1]; C [x·L-1] | Concentration [mol·L-1] is a volume-specific quantity for diluted samples s. In a concentration, the sample is expressed in a variety of formats: count, amount, charge, mass, energy. In solution chemistry, amount concentration is amount of substance nB per volume V of the solution, cB = [B] = nB·V-1 [mol·dm-3] = [mol·L-1]. The standard concentration, c°, is defined as 1 mol·L-1 = 1 M. Count concentration CX = NX·V-1 [x·L-1] is the concentration of the number NX of elementary entities X, for which the less appropriate term 'number concentration' is used by IUPAC. If the sample is expressed as volume Vs (e.g., VO2), then the 'volume-concentration' of Vs in V is termed 'volume fraction', Φs = Vs·V-1 (e.g., volume fraction of O2 in dry air, ΦO2) = 0.20946). Density is the mass concentration in a volume VS of pure sample S. A change of concentration, dcX, in isolated or closed systems at constant volume is due to internal transformations (advancement per volume) only. In closed compressible systems (with a gas phase), the concentration of the gas changes, when pressure-volume work is performed on the system. In open systems, a change of concentration can additionally be due to external flow across the system boundaries. |