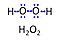Semantic search
| Term | Abbreviation | Description |
|---|---|---|
| Gain | The gain is an amplification factor applied to an input signal to increase the output signal. | |
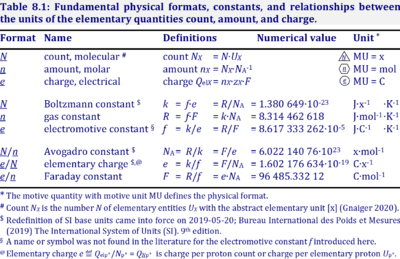
| Gas constant | R [J·mol-1·K-1] | The gas constant, R = 8.314462618 J·mol-1·K-1, has the SI unit for energy per amount per temperature. R is primarily known from the ideal gas equation, pV = nRT or p = cRT. Therefore, RT is the ratio of pressure p and concentration c. R = f·F, the electrochemical constant f times the Faraday constant F. R = k·NA, the Boltzmann constant k times the Avogadro constant NA. |
| Getting started - DatLab | Users have to enter their user details the first time they use DatLab 8 on a specific computer. As well, entering some basic settings is required when connecting DatLab 8 with an O2k for the first time. | |
| Gibbs energy | G [J] | Gibbs energy G [J] is exergy which cannot be created internally (subscript i), but in contrast to internal-energy (diU/dt = 0) is not conserved but is dissipated (diG/dt < 0) in irreversible energy transformations at constant temperature and (barometric) pressure, T,p. Exergy is available as work in reversible energy transformations (100 % efficiency), and can be partially conserved when the exergonic transformation is coupled to an endergonic transformation. |
| Glucose | Glc | Glucose, also known as D-glucose or dextrose, is a monosaccharide and an important carbohydrate in biology. Cells use it as the primary source of energy and a metabolic intermediate. |
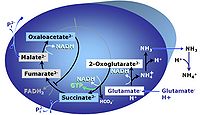
| Glutamate | G | Glutamic acid, C5H9NO4, is an amino acid which occurs under physiological conditions mainly as the anion glutamate-, G, with pKa1 = 2.1, pKa2 = 4.07 and pKa3 = 9.47. Glutamate&malate is a substrate combination supporting an N-linked pathway control state, when glutamate is transported into the mt-matrix via the glutamate-aspartate carrier and reacts with oxaloacetate in the transaminase reaction to form aspartate and oxoglutarate. Glutamate as the sole substrate is transported by the electroneutral glutamate-/OH- exchanger, and is oxidized in the mitochondrial matrix by glutamate dehydrogenase to α-ketoglutarate (2-oxoglutarate), representing the glutamate-anaplerotic pathway control state. Ammonia (the byproduct of the reaction) passes freely through the mitochondrial membrane. |
| Glutamate dehydrogenase | mtGDH | Glutamate dehydrogenase, located in the mitochondrial matrix (mtGDH), is an enzyme that converts glutamate to α-ketoglutarate [1]. mtGDH is not part of the TCA cycle, but is involved in glutaminolysis as an anaplerotic reaction. |
| Glutamate-anaplerotic pathway control state | G | G: Glutamate is an anaplerotic NADH-linked type 4 substrate (N). When supplied as the sole fuel substrate in the glutamate-anaplerotic pathway control state, G is transported by the electroneutral glutamate-/OH- exchanger, and is oxidised via mt-glutamate dehydrogenase in the mitochondrial matrix. The G-pathway plays an important role in glutaminolysis. |
| Glutamate-aspartate carrier | The glutamate-aspartate carrier catalyzes the electrogenic antiport of glutamate- +H+ for aspartate-. It is an important component of the malate-aspartate shuttle in many mitochondria. Due to the symport of glutamate- + +H+, the glutamate-aspartate antiport is not electroneutal and may be impaired by uncoupling. Aminooxyacetate is an inhibitor of the glutamate-aspartate carrier. | |
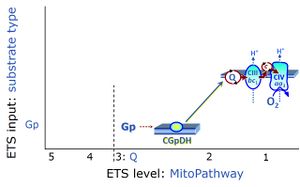
| Glycerophosphate | Gp | Glycerophosphate (synonym: α-glycerophosphate; glycerol-3-phosphate; C3H9O6P) is an organophosphate and it is a component of glycerophospholipids. The mitochondrial Glycerophosphate dehydrogenase Complex oxidizes glycerophosphate to dihydroxyacetone phosphate and feeds electrons directly to ubiquinone. |
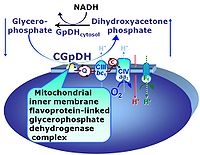
| Glycerophosphate dehydrogenase Complex | CGpDH | Glycerophosphate dehydrogenase complex (CGpDH) is a Complex of the electron transfer-pathway localized at the outer face of the mt-inner membrane. CGpDH is thus distinguished from cytosolic GpDH. CGpDH oxidizes glycerophosphate to dihydroxyacetone phosphate and feeds two electrons into the Q-junction, thus linked to an ET pathway level 3 control state. |
| Glycerophosphate pathway control state | Gp |
The glycerophosphate pathway control state (Gp) is an ET-pathway level 3 control state, supported by the fuel substrate glycerophosphate and electron transfer through glycerophosphate dehydrogenase Complex into the Q-junction. The glycerolphosphate shuttle represents an important pathway, particularly in liver and blood cells, of making cytoplasmic NADH available for mitochondrial oxidative phosphorylation. Cytoplasmic NADH reacts with dihydroxyacetone phosphate catalyzed by cytoplasmic glycerophos-phate dehydrogenase. On the outer face of the inner mitochondrial membrane, mitochondrial glycerophosphate dehydrogenase oxidises glycerophosphate back to dihydroxyacetone phosphate, a reaction not generating NADH but reducing a flavin prosthesic group. The reduced flavoprotein donates its reducing equivalents to the electron transfer-pathway at the level of CoQ. |
| Glycerophosphate shuttle | Gp shuttle |
The glycerophosphate shuttle makes cytoplasmic NADH available for mitochondrial oxidative phosphorylation. Cytoplasmic NADH reacts with dihydroxyacetone phosphate catalyzed by cytoplasmic glycerophosphate dehydrogenase. On the outer face of the inner mitochondrial membrane, glycerophosphate dehydrogenase complex (mitochondrial glycerophosphate dehydrogenase) oxidizes glycerophosphate back to dihydroxyacetone phosphate, a reaction not generating NADH but reducing a flavin prosthesic group. The reduced flavoprotein transfers its reducing equivalents into the Q-junction, thus representing a ET pathway level 3 control state. |
| Gnaiger 2019 MiP2019 | ||
| Graph control - DatLab | A combination of mouse and keyboard commands provides convenient control of graphs in DatLab 8. | |
| Graph layout - DatLab | » See Layout for DatLab graphs. | |
| Graph options - DatLab | Several display options can be applied to a DatLab graph under Graph options. | |
| Group | See population. | |
| H2DCFDA | DCF, H2-DCF | H2DCFDA (dichlorodihydrofluorescein diacetate) is a cell permeant fluorescent probe that has been used as an indicator of ROS presence. It is a reduced form of fluorescein that does not present fluorescence. After entry in the cell, it suffers deacetylation by intracellular esterases, and upon oxidation it is converted to dichlorofluorescein (excitation wavelength ~492–495 nm, emission ~517–527 nm). It may be oxidised by hydrogen peroxide, hydroxyl radical, hypochlorite anion, nitric oxide, peroxyl radical, peroxynitrite, singlet oxygen and superoxide. Has been used as a general indicator of ROS by fluorescence microscopy. |
| HPTS | HPTS | 8-Hydroxypyrene-1,3,6-trisulfonic acid trisodium salt (HPTS) is a ratiometric pH fluorophore; pKa = 7.3. Relative molecular mass: Mr = 524.39 |
| Harmonization | Harmonization is the process of minimizing redundant or conflicting standards which may have evolved independently. To obtain a common basis in reaching a defined objective, critical requirements are identified that need to be retained. | |
| Harmonized European norm | EN-norm | Harmonized European norms are norms valid for all members of the European Union. They are mandatory parts of the individual national collections of norms. |
| Harmonized SUIT protocols | H-SUIT | Harmonized SUIT protocols (H-SUIT) are designed to include cross-linked respiratory states. When performing harmonized SUIT protocols in parallel, measurements of cross-linked respiratory states can be statistically evaluated as replicates across protocols. Additional information is obtained on respiratory coupling and substrate control by including respiratory states that are not common (not cross-linked) across the harmonized protocols. |
| Harmonized standard | A harmonized standard is a European standard developed by a recognized European Standards Organisation: CEN, CENELEC, or ETSI. | |
| Healthy ageing | Healthy ageing: 'WHO has released the first World report on ageing and health, reviewing current knowledge and gaps and providing a public health framework for action. The report is built around a redefinition of healthy ageing that centres on the notion of functional ability: the combination of the intrinsic capacity of the individual, relevant environmental characteristics, and the interactions between the individual and these characteristics' (Beard 2016 The Lancet). | |
| Healthy reference population | HRP | A healthy reference population, HRP, establishes the baseline for the relation between body mass and height in healthy people of zero underweight or overweight, providing a reference for evaluation of deviations towards underweight or overweight and obesity. The WHO Child Growth Standards (WHO-CGS) on height and body mass refer to healthy girls and boys from Brazil, Ghana, India, Norway, Oman and the USA. The Committee on Biological Handbooks compiled data on height and body mass of healthy males from infancy to old age (USA), published before emergence of the fast-food and soft-drink epidemic. Four allometric phases are distinguished with distinct allometric exponents. At heights above 1.26 m/x the allometric exponent is 2.9, equal in women and men, and significantly different from the exponent of 2.0 implicated in the body mass index, BMI [kg/m2]. |
| Heat | Q, Qth [J] | Heat is a form of energy [J]. The relationship between heat and work provides the foundation of thermodynamics, which describes transformations from an initial to a final state of a system. In energy transformations heat may pass through the boundary of the system, at an external heat flow of deQ/dt. |
| Height of humans | h [m]; H [m·x-1] | The height of humans, h, is given in SI units in meters [m]. Humans are countable objects, and the symbol and unit of the number of objects is N [x]. The average height of N objects is, H = h/N [m/x], where h is the heights of all N objects measured on top of each other. Therefore, the height per human has the unit [m·x-1] (compare body mass [kg·x-1]). Without further identifyer, H is considered as the standing height of a human, measured without shoes, hair ornaments and heavy outer garments. |
| Heterothermy | Heterothermy is the variable regulation of body temperature in endotherms which can change their body temperatures as levels of activity and environmental conditions dictate (e.g. hibernators). In regional heterothermy, temperature gradients are present, e.g. between body core and extremeties. | |
| Hexokinase | HK | The hexokinase catalyzes the phosphorylation of D-glucose at position 6 by ATP to yield D-glucose 6-phosphate as well as the phosphorylation of many other hexoses like D-fructose, D-mannose, D-glucosamine. |
| Heymsfield 2014 Am J Clin Nutr | ||
| High signal at zero oxygen | A high signal at zero oxygen may be observed during zero calibration (R0). First, check the quality of the dithionite solution. The following instructions show how to distinguish between a defective sensor head and an electrical leak current. | |
| High-resolution respirometry | HRR | High-resolution respirometry, HRR, is the state-of-the-art approach in mitochondria and cell research to measure respiration in various types of mitochondrial preparations and living cells combined with MultiSensor modules. Mitochondrial function and dysfunction have gained increasing interest, reflecting growing awareness of the fact that mitochondria play a pivotal role in human health and disease. HRR combines instrumental accuracy and reliability with the versatility of applicable protocols, allowing practically unlimited addition and combination of substrates, inhibitors, and uncouplers using the Oroboros O2k-technology. Substrate-uncoupler-inhibitor titration (SUIT) protocols allow the interrogation of numerous mitochondrial pathway and coupling states in a single respirometric assay. Mitochondrial respiratory pathways may be analyzed in detail to evaluate even minor alterations in respiratory coupling and pathway control patterns. The O2k-technology provides sole source instruments, with no other available instrument meeting its specifications for high-resolution respirometry. Technologically, HRR is based on the Oroboros O2k-technology, combining optimized chamber design, application of oxygen-tight materials, electrochemical sensors, Peltier-temperature control, and specially developed software features (DatLab) to obtain the unique sensitive and quantitative resolution of oxygen concentration and oxygen flux, with both, a closed-chamber or open-chamber mode of operation (TIP2k). Standardized calibration of the polarographic oxygen sensor (static sensor calibration), calibration of the sensor response time (dynamic sensor calibration), and evaluation of instrumental background oxygen flux (systemic flux compensation) provide the experimental basis for high accuracy of quantitative results and quality control in HRR. HRR can be extended for MultiSensor analysis by using the O2k-Fluo Smart-Module. Smart Fluo-Sensors are integrated into the O2k to measure simultaneously fluorometric signals using specific fluorophores. Potentiometric modules are available with ion-selective electrodes (pH, TPP+). The PB-Module extends HRR to PhotoBiology with accurate control of the light intensity and measurement of photosynthesis. The O2k and the NextGen-O2k support all these O2k-Modules. The NextGen-O2k all-in-one, however, is unique in supporting Q-Redox and NADH-Redox Modules. |
| Holode | Small entetic units are counted into the reference system on a balance opposite to the experimental system with the large sample, which in balance contains as many abstract units as the count of entetic units in the reference system. | |
| Homeothermy | Homeothermy is the stable regulation of body temperature in endotherms by metabolic heat production and control of heat exchange with the environment, or in ectotherms by behavioural means to select a stable thermal environment. | |
| Hood 2019 Nutr Diabetes | ||
| Horseradish peroxidase | HRP | Horseradish peroxidase readily combines with hydrogen peroxide (H2O2) and the resultant [HRP-H2O2] complex can oxidize a wide variety of hydrogen donors. |
| Hydride | H- | The hydride anion is the species H−. |
| Hydrogen | H2 | Molecular hydrogen H2 is a constituent of the air with a volume fraction of 0.00005. It is a colorless and odorless gas with a molecular mass of 2.016. Its pharmacological potential and effects on mitochondrial metabolism are discussed in various publications without complete evidence on the underlying mechanisms. |
| Hydrogen ion | H+ | The terms hydrogen ion H+ and proton, p or p+, are used synonymously in chemistry. A hydrogen ion is a positively charged molecule. In particle physics, however, a proton is a submolecular and subatomic particle with a positive electric charge. The H+ ion has no electrons and is a bare charge with only about 1/64 000 of the radius of a hydrogen atom. Free H+ is extremely reactive, with an extremely short lifetime in aqueous solutions. There H+ forms the hydronium ion H3O+, which in turn is further solvated by water molecules in clusters such as H5O2+ and H9O4+. The transfer of H+ in an acid–base reaction is referred to as proton transfer. The acid is the H+ donor and the base is the H+ acceptor. |
| Hydrogen ion pump | Mitochondrial hydrogen ion pumps — frequently referred to as "proton pumps" — are large enzyme complexes (CI, CIII, CIV, ATP synthase) spanning the mt-inner membrane mtIM, partially encoded by mtDNA. CI, CIII and CIV are H+ pumps that drive hydrogen ions against the electrochemical protonmotive force pmF and thus generating the pmF, driven by electron transfer from reduced substrates to oxygen. In contrast, ATP synthase (also known as CV) is a H+ pump that utilizes the exergy of proton flow along the protonmotive force to drive phosphorylation of ADP to ATP. | |
| Hydrogen peroxide | H2O2 |
Hydrogen peroxide, H2O2 or dihydrogen dioxide, is one of several reactive oxygen intermediates generally referred to as reactive oxygen species (ROS). It is formed in various enzyme-catalyzed reactions (e.g., superoxide dismutase) with the potential to damage cellular molecules and structures. H2O2 is dismutated by catalase to water and oxygen. H2O2 is produced as a signaling molecule in aerobic metabolism and passes membranes more easily compared to other ROS. |
| Hydrogen sulfide | H2S | Hydrogen sulfide (H2S) is involved in signaling and may have have further biological importance. |
| Hydrogenion flux | JH+ | Volume-specific hydrogenion flux or H+ flux is measured in a closed system as the time derivative of H+ concentration, expressed in units [pmol·s-1·mL-1]. H+ flux can be measured in an open system at steady state, when any acidification of the medium is compensated by external supply of an equivalent amount of base. The extracellular acidification rate (ECAR) is the change of pH in the incubation medium over time, which is zero at steady state. Volume-specific H+ flux is comparable to volume-specific oxygen flux [pmol·s-1·mL-1], which is the (negative) time derivative of oxygen concentration measured in a closed system, corrected for instrumental and chemical background. pH is the negative logarithm of hydrogen ion activity. Therefore, ECAR is of interest in relation to acidification issues in the incubation buffer or culture medium. The physiologically relevant metabolic H+ flux, however, must not be confused with ECAR. |
| Hydron | H+ | Hydron is the general name for the cation H+ used without regard to the nuclear mass of the hydrogen entity (H is the hydro group), either for H in its natural abundance or without distinction between the isotopes. |
| Hydronium ion | H3O+ | H+ forms the hydronium ion H3O+, which in turn is further solvated by water molecules in clusters such as H5O2+ and H9O4+. |
| Hydroxybutyrate | β-hydroxybutyrate or 3-hydroxybutyrate is a ketone body that can be used as a NADH-linked substrate. The β-hydroxybutyrate dehydrogenase produces acetoacetate while reducing NAD+ to NADH.
| |
| Hydroxycinnamate | Hci | Hydroxycinnamate (alpha-cyano-4-hydroxycinnamic acid) is an inhibitor of the pyruvate carrier (0.65 mM). Above 10 mM pyruvate, hydroxycinnamate cannot inhibit respiration from pyruvate, since the weak pyruvic acid can pass the inner mt-membrane in non-dissociated form. |
| Hydroxylamine | Hydroxylamine is an inhibitor of catalase. | |
| Hyperoxia | hyperox | Hyperoxia is defined as environmental oxygen pressure above the normoxic reference level. Cellular and intracellular hyperoxia is imposed on isolated cells and isolated mitochondria at air-level oxygen pressures which are higher compared to cellular and intracellular oxygen pressures under tissue conditions in vivo. Hyperoxic conditions may impose oxidative stress and may increase maximum aerobic performance. |
| Hyperthermia | Hyperthermia in endotherms is a state of stressful up to lethal elevated body core temperature. In humans, the limit of hyperthermia (fever) is considered as >38.3 °C, compared to normothermia at a body temperature of 36.5 to 37.5 °C. | |
| Hyphenation | Hyphenation is used to connect two words (compound words) or two parts of a word to clarify the meaning of a sentence. The same two words may be hyphenated or not depending on context. Hyphenation may present a problem when searching for a term such as 'Steady state'. It is helpful to write 'steady-state measurement', to clarify that the measurement is performed at steady state, rather than implying that a state measurement is steady. But this does not imply that hyphenation is applied to the 'measurement performed at steady state'. Thus, the key word is 'steady state'. Compound adjectives should be hyphenated (steady-state measurement), but if the compound adjective follows the term (measurement at steady state), hyphenation does not add any information and should be avoided. Find more examples and guidelines in the grammarly blog on Hyphen and in apastyle.apa.org. | |
| Hypothermia | Hypothermia in endotherms is a state of stressful up to lethal low body core temperature. In humans, the limit of hypothermia is considered as 35 °C, compared to normothermia at a body temperature of 36.5 to 37.5 °C. Hypothermia is classified as mild (32–35 °C), moderate (28–32 °C), severe (20–28 °C), and profound (<20 °C). | |
| Hypoxia | hypox | Hypoxia (hypox) is defined in respiratory physiology as the state when insufficient O2 is available for respiration, compared to environmental hypoxia defined as environmental oxygen pressures below the normoxic reference level. Three major categories of hypoxia are (1) environmental hypoxia, (2) physiological tissue hypoxia in hyperactivated states (e.g. at VO2max) with intracellular oxygen demand/supply balance at steady state in tissues at environmental normoxia, compared to tissue normoxia in physiologically balanced states, and (3) pathological tissue hypoxia including ischemia and stroke, anaemia, chronic heart disease, chronic obstructive pulmonary disease, severe COVID-19, and obstructive sleep apnea. Pathological hypoxia leads to tissue hypoxia and heterogenous intracellular anoxia. Clinical oxygen treatment ('environmental hyperoxia') may not or only partially overcome pathological tissue hypoxia. |
| IRDiRC | IRDiRC |  The International Rare Diseases Research Consortium (IRDiRC) teams up researchers and organizations investing in rare diseases research in order to achieve two main objectives by the year 2020, namely to deliver 200 new therapies for rare diseases and means to diagnose most rare diseases. The International Rare Diseases Research Consortium (IRDiRC) teams up researchers and organizations investing in rare diseases research in order to achieve two main objectives by the year 2020, namely to deliver 200 new therapies for rare diseases and means to diagnose most rare diseases. |
| ISE Package 1 TPP or Ca | O2k-TPP+ and Ca2+ ISE\1 Chamber: ISE-Package for 1 TPP+ and Ca2+ electrode. | |
| ISE-Ca2+ Membranes | ISE-Ca2+ Membranes: PVC, 4 mm diameter, box of 5 membranes. To be used with the O2k-TPP+ ISE-Module. | |
| ISE-Compressible Tube | ISE-Compressible Tube for Ion-Selective Electrode TPP+ and Ca2+. | |
| ISE-Filling Syringe | ISE-Filling Syringe with needle for Ion-Selective Electrode TPP+ and Ca2+. | |
| ISE-Inner Glass Electrode | ISE-Inner Glass Electrode of ISE, with Ag/AgCl- and Pt-wire | |
| ISE-Membrane Mounting Tool | ISE-Membrane Mounting Tool for Ion-Selective Electrode TPP+ and Ca2+. O2k-TPP+ ISE-Module: mounting tool included. | |
| ISE-Membrane Seal | ISE-Membrane Seal for Ion-Selective Electrode TPP+ and Ca2+. | |
| ISE-TPP+ Membranes | ISE-TPP+ Membranes, PVC, 4 mm diameter, box of 5 membranes. | |
| ISO 10012:2003 Measurement management systems | ISO 10012:2003 | ISO 10012:2003 Measurement management systems — Requirements for measurement processes and measuring equipment: An effective measurement management system ensures that measuring equipment and measurement processes are fit for their intended use and is important in achieving product quality objectives and managing the risk of incorrect measurement results. The objective of a measurement management system is to manage the risk that measuring equipment and measurement processes could produce incorrect results affecting the quality of an organization’s product. The methods used for the measurement management system range from basic equipment verification to the application of statistical techniques in the measurement process control. |
| ISO 13528:2015 Statistical methods for use in proficiency testing by interlaboratory comparison | ISO 13528:2015 | ISO 13528:2015 Statistical methods for use in proficiency testing by interlaboratory comparison: Proficiency testing involves the use of interlaboratory comparisons to determine the performance of participants (which may be laboratories, inspection bodies, or individuals) for specific tests or measurements, and to monitor their continuing performance. There are a number of typical purposes of proficiency testing ISO/IEC 17043:2010. These include the evaluation of laboratory performance, the identification of problems in laboratories, establishing effectiveness and comparability of test or measurement methods, the provision of additional confidence to laboratory customers, validation of uncertainty claims, and the education of participating laboratories. The statistical design and analytical techniques applied must be appropriate for the stated purpose(s). |
| ISO 15189:2012 Medical laboratories — Particular requirements for quality and competence | ISO 15189:2012 | ISO 15189:2012 Medical laboratories — Particular requirements for quality and competence: This International Standard is for use by medical laboratories in developing their quality management systems and assessing their own competence, and for use by accreditation bodies in confirming or recognising the competence of medical laboratories. While this International Standard is intended for use throughout the currently recognised disciplines of medical laboratory services, those working in other services and disciplines could also find it useful and appropriate. |
| ISO 17511:2003 In vitro diagnostic medical devices | ISO 17511:2003 | ISO 17511:2003 In vitro diagnostic medical devices -- Measurement of quantities in biological samples -- Metrological traceability of values assigned to calibrators and control materials: For measurements of quantities in laboratory medicine, it is essential that the quantity is adequately defined and that the results reported to the physicians or other health care personel and patients are adequately accurate (true and precise) to allow correct medical interpretation and comparability over time and space. |
| ISO 9001:2015 Quality management systems - requirements | ISO 9001:2015 | ISO 9001:2015 Quality management systems - requirements: The adoption of a quality management system is a strategic decision for an organization that can help to improve its overall performance and provide a sound basis for sustainable development initiatives. Consistently meeting requirements and addressing future needs and expectations poses a challenge for organizations in an increasingly dynamic and complex environment. To achieve this objective, the organization might find it necessary to adopt various forms of improvement in addition to correction and continual improvement, such as breakthrough change, innovation and re-organization. |
| ISO/IEC 17025:2005 Competence of testing and calibration laboratories | ISO/IEC 17025:2005 | ISO/IEC 17025:2005 General requirements for the competence of testing and calibration laboratories: The use of this International Standard will facilitate cooperation between laboratories and other bodies, and assist in the exchange of information and experience, and in the harmonization of standards and procedures. This International Standard specifies the general requirements for the competence to carry out tests and/or calibrations, including sampling. It covers testing and calibration performed using standard methods, non-standard methods, and laboratory-developed methods. |
| ISO/IEC 17043:2010 General requirements for proficiency testing | ISO/IEC 17043:2010 | ISO/IEC 17043:2010 Conformity assessment — General requirements for proficiency testing: The use of interlaboratory comparisons is increasing internationally. This International Standard provides a consistent basis to determine the competence of organizations that provide proficiency testing. |
| ISS-Filter and Tubing | ISS-Filter and Tubing, ISS-Integrated Suction System. | |
| ISS-Integrated Suction System | ISS-Integrated Suction System: Suction pump with stainless steel housing, 2 liter waste bottle, filter and tubing; for siphoning off excess medium from the O2k-Stopper and for emptying the O2k-chambers. The ISS is included as a standard component of the O2k-FluoRespirometer. Media containing living cells or microorganisms, various poisons (inhibitors, uncouplers) and mixtures of proteins and substrates are safely disposed off in the 2-litre waste bottle. | |
| ISS-Lid | ISS-Lid, for ISS-Waste Bottle, component of the ISS-Integrated Suction System. | |
| ISS-Steel Housing | ISS-Steel Housing, a component of the ISS-Integrated Suction System. | |
| ISS-Waste Bottle | ISS-Waste Bottle, 2-liter, component of the ISS-Integrated Suction System. | |
| Iconic symbols | Iconic symbols are used in ergodynamics to indicate more explicitely — compared to standard SI or IUPAC symbols — the quantity represented and some boundary conditions. This is particularly the case in normalized quantities (ratios of quantities). Iconic (or canonical) symbols help to clarify the meaning, are based on SI and IUPAC symbols as far as possible, and may be translated into more commonly used, practical symbols. Several ambiguities in SI and IUPAC symbols are eliminated by the systematic structure of iconic symbols, but it may be impossible to avoid all ambiguities, particulary when long (canonical) symbols are abbreviated in a particular context. Clarity is improved always by showing the unit of a quantity together with the symbol of the quantity. Iconic symbols cannot be identical with IUPAC symbols when a different definition is used — this would add to the confusion. For example, the IUPAC symbols nB [mol] and VB [m3] denote amount and volume of B. Consequently, it should be expected, that the symbol QB indicates charge of B [C]. However, the IUPAC symbol QB is used for particle charge per ion B [C·x-1]. This prohibits a consistent definition of QB as a potential iconic symbol for charge carried by a given quantity of ions B with unit [C], instead of particle charge per ion B with unit [C·x-1]. Hence, the conventional ambigous system forces compatible iconic symbols to be more complicated, using QelB [C] and QNB [C·x-1] to distinguish charge of B from charge per elementary B. QnB [C·mol-1] is charge per molar amount of B. | |
| Illumination | F10 | The chambers of the Oroboros O2k are illuminated by an internal LED. The illumination is switched on and off in DatLab during the experiment by pressing [F10]. This illumination must be distinguished from light introduced into the chambers by LEDs for the purpose of spectrophotometric and fluorometric measurements. For these, the internal illumination must be switched off. |
| Illumination on/off | F10 | The illumination in both chambers is switched on/off. |
| Impact factor | IF | Impact factor is a measure of a scientific journal's citations per publication. The Journal Citation Reports, maintained by Clarivate Analytics, provides the calculated impact factors. The IF is frequently used as an indicator of a journal's importance or prestige, which is nowadays increasingly contested. |
| Improvement score | RIS | The relative improvement score, RIS, provides a measure of improvement of a trait from a value measured at baseline, B, to a value measured after treatment, T, expressing the total improvement, T-B, in relation to the theoretical scope of improvement and the level of the trait observed at baseline. 'RIS incorporates the concept of diminishing returns and consideres maintaining a high value of a trait as an improvement relative to the potential loss. |
| In vitro diagnostic medical device | IVD | A medical device is an in vitro diagnostic medical device (IVD) if it is a reagent, calibrator, control material, kit, specimen receptacle, software, instrument, apparatus, equipment or system, whether used alone or in combination with other diagnostic goods for in vitro use. |
| Incident light | The term incident light is used for a beam of light falling upon a surface. | |
| Inclusion criteria | The Inclusion criteria are based on key features of the target population that the researchers will use to answer their question. These criteria should identify the study population in a consistent, reliable, uniform, and objective manner. With the Exclusion criteria, this factor must be a cofounder for the outcome parameter | |
| Indian Academy of Pediatrics Growth Charts Committee 2015 Indian Pediatr | ||
| Inorganic phosphate | Pi | Inorgnic phosphate (Pi) is a salt of phosphoric acid. In solution near physiological pH, the species HPO42- and H2PO4- dominate. See also: Phosphate carrier (Pic). |
| Inside the O2k | A glance inside the Oroboros O2k | |
| Install Oroboros protocol package | The standard Instrumental and SUIT DL-Protocols package is automatically implemented with the simple DatLab programme installation. We recommend a 'clean install': rename your previous DatLab programme subdirectory (e.g. C:\DatLab_OLD). Updates and newly developed DL protocols can be simply downloaded by clicking on [Protocols]\Install Oroboros protocol package. | |
| Instrumental: Browse DL-Protocols and templates | Instrumental DL-Protocols (DLP) including DatLab example traces, instructions, brief explanatory texts, links to relevant pages and templates for data evaluation can be browsed from inside DatLab 7.4. Click on menu [Protocols]\Instrumental: Browse DL-Protocols and templates to open a folder with all the DL-Protocols and templates for cleaning, calibration, and background determination provided with the DatLab 7.4. Select a sub-directory and open an DL-Protocol and/or template as desired. | |
| Integration time | Integration time is the time taken to scan a single full range spectrum using photodiode arrays. It is equivalent to the exposure time for a camera. The shortest integration time defines the fastest response time of a spectrophotometer. Increasing the integration time increases the sensitivity of the device. The white balance or balance and subsequent measurements must always be carried out at the same integration time. | |
| Intensive quantity | Intensive quantities are partial derivatives of an extensive quantity by the advancement, dtrξX, of an energy transformation tr; example: Force. In contrast to extensive quantities which pertain to the entire system and are additive, extensive quantities 'take well defined values at each point of the system' (Prigogine 1967 Interscience) and are non-additive. Intensive and extensive quantities can be easily discriminated by the units, e.g. [J] for the extensive quantity, in contrast to [J·mol-1] for the corresponding intensive quantity. In the general definition of thermodynamics, intensive quantities are not distinguished from specific quantities (Cohen 2008 IUPAC Green Book). Ergodynamics emphasizes the contrast between specific quantities which are extensive quantities normalized for a variable expressing system size (mass, volume of the system, amount of substance in a system) and intensive quantities which are normalized for the motive unit of a transformation (mass exchanged, volume change of the system, amount of substance reacting in a system; Gnaiger 1993 Pure Appl Chem). Intensive and specific quantities are both non-additive, take well defined values at each point of the system, and both corresponding quantities are expressed in identical units, e.g. the intensive quantity Gibbs force of a catabolic reaction (such as oxidation; 0 = -1 Glc - 6 O2 + 6 CO2 + 6 H2O), ΔkGGlc [kJ·mol-1], and the specific quantity Gibbs energy per mole glucose contained in a system, GGlc [kJ·mol-1] (with respect to an arbitrarily defined reference state, such as the reference state of formation or combustion). | |
| Interlaboratory comparison | An interlaboratory comparison is the organization, performance and evaluation of measurements or tests on the same or similar items by two or more laboratories in accordance with predetermined conditions. | |
| Internal flow | Ii [MU·s-1] | Within the system boundaries, irreversible internal flows, Ii,—including chemical reactions and the dissipation of internal gradients of heat and matter—contribute to internal entropy production, diS/dt. In contrast, external flows, Ie, of heat, work, and matter proceed reversibly across the system boundaries (of zero thickness). Flows are expressed in various formats per unit of time, with corresponding motive units [MU], such as chemical [mol], electrical [C], mass [kg]. Flow is an extensive quantity, in contrast to flux as a specific quantity. |
| Internal-energy | U [J] | Internal-energy, U [J], can neither be destroyed nor created (first law of thermodynamics: diU/dt = 0). Note that internal (subscript i), as opposed to external (subscript e), must be distinguished from "internal-energy", U, which contrasts with "Helmholtz energy", A, as enthalpy, H, contrasts with Gibbs energy, G. |
| International Mito Patients (IMP) | IMP |  The International Mito Patients is a network of national patient organizations involved in mitochondrial disease. Mitochondrial disease is a rare disease with a limited number of patients per country. The national patient organizations which are a member of IMP each are active and powerful in their own countries. By joining forces IMP can represent a large group of patients and as such be their voice on an international level. The International Mito Patients is a network of national patient organizations involved in mitochondrial disease. Mitochondrial disease is a rare disease with a limited number of patients per country. The national patient organizations which are a member of IMP each are active and powerful in their own countries. By joining forces IMP can represent a large group of patients and as such be their voice on an international level. |
| International Society for Mountain Medicine | ISMM | The International Society for Mountain Medicine is an interdisciplinary society comprising about xx members worldwide. Its purpose is .. |
| International Society on Oxygen Transport to Tissue | ISOTT |
The International Society on Oxygen Transport to Tissue is an interdisciplinary society comprising about 250 members worldwide. Its purpose is to further the understanding of all aspects of the processes involved in the transport of oxygen from the air to its ultimate consumption in the cells of the various organs of the body. Founded in 1973, the society has been the leading platform for the presentation of many of the technological and conceptual developments within the field both at the meetings themselves and in the proceedings of the society. |
| International Standard Serial Number | ISSN | The International Standard Serial Number, ISSN, is a code used to identify periodical publications, independent of which media are used (print and/or electronic). - Bioenergetics Communications, BEC: ISSN 2791-4690 |
| International System of Units | SI | The International System of Units (SI) is the modern form of the metric system of units for use in all aspects of life, including international trade, manufacturing, security, health and safety, protection of the environment, and in the basic science that underpins all of these. The system of quantities underlying the SI and the equations relating them are based on the present description of nature and are familiar to all scientists, technologists and engineers. The definition of the SI units is established in terms of a set of seven defining constants. The complete system of units can be derived from the fixed values of these defining constants, expressed in the units of the SI. These seven defining constants are the most fundamental feature of the definition of the entire system of units. These particular constants were chosen after having been identified as being the best choice, taking into account the previous definition of the SI, which was based on seven base units, and progress in science (p. 125). |
| International Union of Pure and Applied Chemistry, IUPAC | IUPAC | The International Union of Pure and Applied Chemistry (IUPAC) celebrated in 2019 the 100th anniversary, which coincided with the International Year of the Periodic Table of Chemical Elements (IYPT 2019). IUPAC {Quote} notes that marking Mendeleev's achievement will show how the periodic table is central to connecting cultural, economic, and political dimensions of global society “through a common language” {end of Quote} (Horton 2019). 2019 is proclaimed as the International Year of the Periodic Table of Chemical Elements (IYPT 2019). For a common language in mitochondrial physiology and bioenergetics, the IUPAC Green book (Cohen et al 2008) is a most valuable resource, which unfortunately is largely neglected in bioenergetics textbooks. Integration of open systems and non-equilibrium thermodynamic approaches remains a challenge for developing a common language (Gnaiger 1993; BEC 2020.1). |
| International oxygraph course | IOC | International Oxygraph Course (IOC), see O2k-Workshops. |