Semantic search
| Term | Abbreviation | Description |
|---|---|---|
| % | % | The symbol % indicates 'per cent' (per hundred). {Quote} The internationally recognized symbol % (per cent) may be used with the SI. When it is used, a space separates the number and the symbol %. {end of Quote}. |
| 1OctM;2D;3PG;4S;5U;6Rot- | FNS(Oct,PGM) | 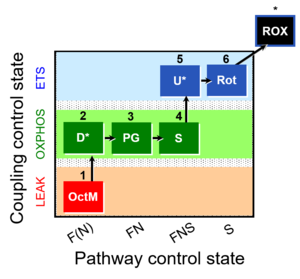 |
| 1PGM;2D;3S;4Rot;5U- | NS(PGM) | 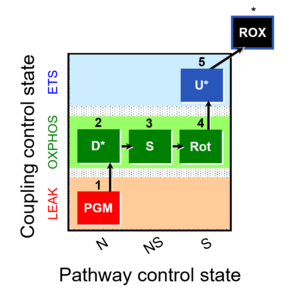 |
| 1PGM;2D;3U;4S;5Rot- | NS(PGM) | 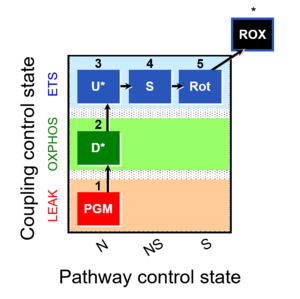 |
| 1PM;2D;3G;4U;5S;6Rot- | NS(PGM) | 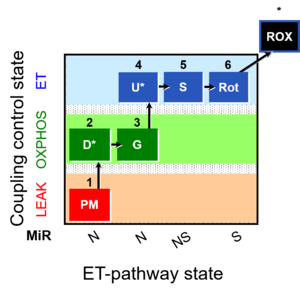 |
| 2-Deoxyglucose | 2-DG | 2-Deoxyglucose, also known as 2-deoxy-D-glucose is a glucose derivative that has the 2-hydroxyl group replaced by hydrogen. It competitively inhibits glycolysis by blocking hexokinase and phosphohexoseisomerase. |
| 2-Hydroxyglutarate | 2HG | Reduction of oxoglutarate (2OG or alpha-ketoglutarate) to 2-hydroxyglutarate (2HG) is driven by NADPH. 2HG is also formed in side reactions of lactate dehydrogenase and malate dehydrogenase. Millimolar 2HG concentrations are found in some cancer cells compared to , whereas side activities of lactate and malate dehydrogenase form submillimolar s-2-hydroxyglutarate (s-2HG). However, even wild-type IDH1 and IDH2, notably under shifts toward reductive carboxylation glutaminolysis or changes in other enzymes, lead to “intermediate” 0.01–0.1 mM 2HG levels, for example, in breast carcinoma compared with nanomolar concentrations in benign cells. 2HG is considered an important player in reprogramming metabolism of cancer cells. |
| 2-mercaptoacetate | 2-mercaptoacetate is an inhibitor of medium-chain acyl-CoA dehydrogenase, MCAD, the rate-limiting enzyme of octanoylcarnitine oxidation. 2-mercaptoacetate has been used as an inhibitor of fatty acid oxidation (F-pathway control state). In permeabilized rat soleus muscle fibers, pre-incubation with 1 mM 2-mercaptoacetate for 45 min resulted in 58% inhibition of MCAD and decreased octanoylcarnitine&malate stimulated respiration by approximately 60% (Osiki 2016 FASEB J). | |
| 3-Mercaptopropionic acid | MPA | 3-Mercaptopropionic acid (MPA) inhibits long chain acyl-CoA dehydrogenases (ACADs). |
| ADP | D | Adenosine diphosphate is a nucleotide. In OXPHOS core metabolism, ADP is a substrate of ANT and ATP synthase in the phosphorylation system. ADP is the discharged or low-energy counterpart of ATP. ADP can accept chemical energy by regaining a phosphate group to become ATP, in substrate-level phosphorylation (in anaerobic catabolism), at the expense of solar energy (in photosynthetic cells) or chemiosmotic energy (respiration in heterotrophic cells). ADP is added to mitochondrial preparations at kinetically saturating concentrations to induce the active state for evaluation of OXPHOS capacity. |
| AMPK | AMPK | AMP-activated protein kinase is a regulatory protein which acts as crucial cellular energy sensor by sensing AMP, ADP and/or Ca2+ levels in response to metabolic stresses or drug administration. |
| ASAPbio | Science only progresses as quickly and efficiently as it is shared. But even with all of the technological capabilities available today, the process of publishing scientific work is taking longer than ever. ASAPbio (Accelerating Science and Publication in biology) is a scientist-driven nonprofit working to address this problem by promoting innovation and transparency in life sciences communication. In 2015, ASAPbio founder Ron Vale published an analysis of the increasing time to first-author publication among graduate students at UCSF, and proposed a more widespread use of preprints in the life sciences as a potential solution. | |
| ATP | T | Adenosine triphosphate is a nucleotid and functions as the major carrier of chemical energy in the cells. As it transfers its energy to other molecules, it looses its terminal phosphate group and becomes adenosine diphosphate (ADP). |
| ATP synthase | CV | ATP synthase or F-ATPase (F1FO-ATPase; the use of Complex V is discouraged) catalyzes the endergonic phosphorylation of ADP to ATP in an over-all exergonic process that is driven by proton translocation along the protonmotive force. The ATP synthase can be inhibited by oligomycin. |
| ATPases | ATPases are enzymes that hydrolyse ATP, releasing ADP and inorganic phosphate. The contamination of isolated mitochondria with ATPases from other organelles and endogenous adenylates can lead to the production of ADP, which can stimulate respiration. This situation would lead to an overestimation of LEAK respiration measured in the absence of ADP, L(n) and subsequent inhibition of respiration by oligomycin, L(Omy). | |
| Abscissa | x | The abscissa is the horizontal axis x of a rectangular two-dimensional graph with the ordinate y as the vertical axis. Values X are placed horizontally from the origin. |
| Absorbance | A | Also known as attenuation or extinction, absorbance (A) is a measure of the difference between the incident light intensity (I0) and the intensity of light emerging from a sample (I). It is defined as: A = log (I0/I) |
| Absorbance spectrum | When light enters a sample, the amount of light that it absorbs is dependent upon the wavelength of the incident light. The absorbance spectrum is the curve derived by plotting the measured absorbance against the wavelength of the light emerging from the sample over a given wavelength range. An absorbance spectrum may be characterised by peaks and troughs (absorbance maxima and minima) that can be used to identify, and sometimes quantify, different absorbing substances present in a sample. | |
| Absorption | Abs | When light enters a sample and emerges with an intensity (I), absorption (Abs) is the fraction of the light absorbed by the sample compared with the incident light intensity (I0): Abs = 1-I/I0. Absorption can also be expressed as Abs = 1-T, where T is the transmittance. |
| Absorption spectrum | An absorption spectrum is similar to an absorbance spectrum of a sample, but plotted as a function of absorption against wavelength. | |
| Abundance | In chemistry or physics, abundance or natural abundance refers to the amount of a chemical element isotope existing in nature. The abundance of an isotope on the Earth may vary depending on the place, but remains relatively constant in time (on a short-term scale). In a chemical reaction, the reactant is in abundance when the quantity of a substance is enough (or high) and constant during the reaction. Relative abundance represents the percentage of the total amount of all isotopes of the element. The relative abundance of each isotope in a sample can be identified using mass spectrometry. | |
| Acceleration | a, g [m·s-2] | Acceleration, a, is the change of velocity over time [m·s-2]. a = dv/dt The symbol g is used for acceleration of free fall. The standard acceleration of free fall is defined as gn = 9.80665 [m·s-2]. |
| Acclimation | Acclimation is an immediate time scale adaption expressing phenotypic plasticity in response to changes of a single variable under controlled laboratory conditions. | |
| Acclimatization | Acclimatization is an immediate time scale adaption expressing phenotypic plasticity in response to changes of habitat conditions and life style where several variables may change simultaneously. | |
| Accuracy | The accuracy of a method is the degree of agreement between an individual test result generated by the method and the true value. | |
| Acetyl-CoA | Acetyl-CoA, C23H38N7O17P3S, is a central piece in metabolism involved in several biological processes, but its main role is to deliver the acetyl group into the TCA cycle for its oxidation. It can be synthesized in different pathways: (i) in glycolysis from pyruvate, by pyruvate dehydrogenase, which also forms NADH; (ii) from fatty acids β-oxidation, which releases one acetyl-CoA each round; (iii) in the catabolism of some amino acids such as leucine, lysine, phenylalanine, tyrosine and tryptophan.
| |
| Aconitase | Aco | Aconitase is a TCA cycle enzyme that catalyzes the reversible isomerization of citrate to isocitrate. Also, an isoform is also present in the cytosol acting as a trans-regulatory factor that controls iron homeostasis at a post-transcriptional level. |
| Activity | a | The activity (relative activity) is a dimensionless quantity related to the concentration or partial pressure of a dissolved substance. The activity of a dissolved substance B equals the concentration, cB [mol·L-1], at high dilution divided by the unit concentration, c° = 1 mol·L-1: aB = cB/c° This simple relationship applies frequently to substances at high dilutions <10 mmol·L-1 (<10 mol·m-3). In general, the concentration of a solute has to be corrected for the activity coefficient (concentration basis), γB, aB = γB·cB/c° At high dilution, γB = 1. In general, the relative activity is defined by the chemical potential, µB aB = exp[(µB-µ°)/RT] |
| Acyl-CoA dehydrogenase | ACAD | Acyl-CoA dehydrogenases ACADs are localized in the mitochondrial matrix. Several ACADs are distinguished: short-chain (SCAD), medium-chain (MCAD), and long-chain (LCAD). ACAD9 is expressed in human brain. ACADs catalyze the reaction
|
| Acyl-CoA oxidase | Acyl-CoA oxidase is considered as a rate-limiting step in peroxysomal β-oxidation, which carries out few β-oxidation cycles, thus shortening very-long-chain fatty acids (>C20). Electrons are directly transferred from FADH2 to O2 with the formation of H2O2. | |
| Acylcarnitine | AC | Acylcarnitines are esters derivative of carnitine and fatty acids, involved in the metabolism of fatty acids. Long-chain acylcarnitines such as palmitoylcarnitine must be transported in this form, conjugated to carnitine, into the mitochondria to deliver fatty acids for fatty acid oxidation and energy production. Medium-chain acylcarnitines such as octanoylcarnitine are also frequently used for high-resolution respirometry. |
| Adaptation | Adaptation is an evolutionary time scale expression of phenotypic plasticity in response to selective pressures prevailing under various habitat conditions. | |
| Add Graph/Delete bottom graph | Add: A new graph is added at the bottom of the screen. Select plots for display in the new graph, Ctrl+F6. Delete: Delete one of the graphs displayed in DatLab. | |
| Additive effect of convergent electron flow | Aα&β | Additivity Aα&β describes the principle of substrate control of mitochondrial respiration with convergent electron flow. The additive effect of convergent electron flow is a consequence of electron flow converging at the Q-junction from respiratory Complexes I and II (NS or CI&II e-input). Further additivity may be observed by convergent electron flow through glycerophosphate dehydrogenase and electron-transferring flavoprotein Complex. Convergent electron flow corresponds to the operation of the TCA cycle and mitochondrial substrate supply in vivo. Physiological substrate combinations supporting convergent NS e-input are required for reconstitution of intracellular TCA cycle function. Convergent electron flow simultaneously through Complexes I and II into the Q-junction supports higher OXPHOS capacity and ET capacity than separate electron flow through either CI or CII. The convergent NS effect may be completely or partially additive, suggesting that conventional bioenergetic protocols with mt-preparations have underestimated cellular OXPHOS-capacities, due to the gating effect through a single branch. Complete additivity is defined as the condition when the sum of separately measured respiratory capacities, N + S, is identical to the capacity measured in the state with combined substrates, NS (CI&II). This condition of complete additivity, NS=N+S, would be obtained if electron channeling through supercomplex CI, CIII and CIV does not interact with the pool of redox intermediates in the pathway from CII to CIII and CIV, and if the capacity of the phosphorylation system does not limit OXPHOS capacity (excess E-P capacity factor is zero). In most cases, however, additivity is incomplete, NS < N+S. |
| Adenine nucleotide translocase | ANT | The adenine nucleotide translocator, ANT, exchanges ADP for ATP in an electrogenic antiport across the inner mt-membrane. The ANT is inhibited by atractyloside, carboxyatractyloside and bongkrekik acid. The ANT is a component of the phosphorylation system. |
| Adenine nucleotides | AN | Adenine nucleotides, which are also sometimes referred to as adenosines or adenylates, are a group of organic molecules including AMP, ADP and ATP. These molecules present the major players of energy storage and transfer. |
| Adenylate kinase | ADK | Adenylate kinase, which is also called myokinase, is a phosphotransferase enzyme that is located in the mitochondrial intermembrane space and catalyzes the rephosphorylation of AMP to ADP in the reaction ATP + AMP ↔ ADP + ADP. |
| Advancement | dtrξ [MU] | In an isomorphic analysis, any form of flow is the advancement of a process per unit of time, expressed in a specific motive unit [MU∙s-1], e.g., ampere for electric flow or current, Iel = delξ/dt [A≡C∙s-1], watt for thermal or heat flow, Ith = dthξ/dt [W≡J∙s-1], and for chemical flow of reaction, Ir = drξ/dt, the unit is [mol∙s-1] (extent of reaction per time). The corresponding motive forces are the partial exergy (Gibbs energy) changes per advancement [J∙MU-1], expressed in volt for electric force, ΔelF = ∂G/∂elξ [V≡J∙C-1], dimensionless for thermal force, ΔthF = ∂G/∂thξ [J∙J-1], and for chemical force, ΔrF = ∂G/∂rξ, the unit is [J∙mol-1], which deserves a specific acronym [Jol] comparable to volt [V]. For chemical processes of reaction (spontaneous from high-potential substrates to low-potential products) and compartmental diffusion (spontaneous from a high-potential compartment to a low-potential compartment), the advancement is the amount of motive substance that has undergone a compartmental transformation [mol]. The concept was originally introduced by De Donder [1]. Central to the concept of advancement is the stoichiometric number, νi, associated with each motive component i (transformant [2]). In a chemical reaction r the motive entity is the stoichiometric amount of reactant, drni, with stoichiometric number νi. The advancement of the chemical reaction, drξ [mol], is defined as, drξ = drni·νi-1 The flow of the chemical reaction, Ir [mol·s-1], is advancement per time, Ir = drξ·dt-1 This concept of advancement is extended to compartmental diffusion and the advancement of charged particles [3], and to any discontinuous transformation in compartmental systems [2], |
| Advancement per volume | dtrY [MU∙L-1] | Advancement per volume or volume-specific advancement, dtrY, is related to advancement of a transformation, dtrY = dtrξ∙V-1 [MU∙L-1]. Compare dtrY with the amount of substance j per volume, cj (concentration), related to amount, cj = nj∙V-1 [mol∙V-1]. Advancement per volume is particularly introduced for chemical reactions, drY, and has the dimension of concentration (amount per volume [mol∙L-1]). In an open system at steady-state, however, the concentration does not change as the reaction advances. Only in closed systems and isolated systems, specific advancement equals the change in concentration divided by the stoichiometric number, drY = dcj/νj (closed system) drY = drcj/νj (general) With a focus on internal transformations (i; specifically: chemical reactions, r), dcj is replaced by the partial change of concentration, drcj (a transformation variable or process variable). drcj contributes to the total change of concentration, dcj (a system variable or variable of state). In open systems at steady-state, drcj is compensated by external processes, decj = -drcj, exerting an effect on the total concentration change of substance j, dcj = drcj + decj = 0 (steady state) dcj = drcj + decj (general) |
| Advantage of preprints | The advantages of preprints, the excitement and concerns about the role that preprints can play in disseminating research findings in the life sciences are discussed by N Bhalla (2016). | |
| Aerobic | ox | The aerobic state of metabolism is defined by the presence of oxygen (air) and therefore the potential for oxidative reactions (ox) to proceed, particularly in oxidative phosphorylation (OXPHOS). Aerobic metabolism (with involvement of oxygen) is contrasted with anaerobic metabolism (without involvement of oxygen): Whereas anaerobic metabolism may proceed in the absence or presence of oxygen (anoxic or oxic conditions), aerobic metabolism is restricted to oxic conditions. Below the critical oxygen pressure, aerobic ATP production decreases. |
| Affinity of reaction | A [J·mol-1] | The concept of affinity and hence chemical force is deeply rooted in the notion of attraction (and repulsion) of alchemy, which was the foundation of chemistry originally, but diverted away from laboratory experiments towards occult secret societies [1].** Newton's extensive experimental alchemical work and his substantial written track record on alchemy (which he did not publish) is seen today as a key inspiration for his development of the concept of the gravitational force [2-4]. This marks a transition of the meaning of affinity, from the descriptive 'adjacent' (proximity) to the causative 'attractive' (force) [5]. Correspondingly, Lavoisier (1790) equates affinity and force [6]: “... the degree of force or affinity with which the acid adheres to the base” [5]. By discussing the influence of electricity and gravity on chemical affinity, Liebig (1844) considers affinity as a force [7]. This leads to Guldberg and Waage's mass action ratio ('Studies concerning affinity', 1864; see [5]), the free energy and chemical affinity of Helmholtz (1882 [8]), and chemical thermodynamics of irreversible processes [9], where flux-force relations are center stage [10]. According to the IUPAC definition, the affinity of reaction, A [J·mol-1], equals the negative molar Gibbs energy of reaction [11], which is the negative Gibbs force of reaction (derivative of Gibbs energy per advancement of reaction [12]): -A = ΔrF = ∂G/∂rξ The historical account of affinity is summarized by concluding, that today affinity of reaction should be considered as an isomorphic motive force and be generalized as such. This will help to (1) avoid confusing reversals of sign conventions (repulsion = negative attraction; pull = negative push), (2) unify symbols across classical and nonequilibrium thermodynamics [12,13], and thus (3) facilitate interdisciplinary communication by freeing ourselves from the alchemical, arcane scientific nomenclature. |
| Air calibration | R1 | Air calibration of an oxygen sensor (polarographic oxygen sensor) is performed routinely on any day before starting a respirometric experiment. The volume fraction of oxygen in dry air is constant. An aqueous solution in equilibrium with air has the same partial pressure as that in water vapour saturated air. The water vapour is a function of temperature only. The partial oxygen pressure in aqueous solution in equilibrium with air is, therefore, a function of total barometric pressure and temperature. Bubbling an aqueous solution with air generates deviations from barometric pressure within small gas bubbles and is, therefore, not recommended. To equilibrate an aqueous solution ata known partial pressure of oxygen [kPa], the aqueous solution is stirred rigorously in a chamber enclosing air at constant temperature. The concentration of oxygen, cO2 [µM], is obtained at any partial pressure by multiplying the partial pressure by the oxygen solubility, SO2 [µM/kPa]. SO2 is a function of temperature and composition of the salt solution, and is thus a function of the experimental medium. The solubility factor of the medium, FM, expresses the oxygen solubility relative to pure water at any experimental temperature. FM is 0.89 in serum (37 °C) and 0.92 in MiR06 or MiR05 (30 °C and 37 °C). |
| Allegations of research misconduct | Allegations of research misconduct are handled with care. Publishers and editors shall take reasonable steps to identify and prevent the publication of papers where research misconduct has occurred, including plagiarism, citation manipulation, and data falsification/fabrication, among others. In no case shall a journal or its editors encourage such misconduct, or knowingly allow such misconduct to take place. In the event that a journal's publisher or editors are made aware of any allegation of research misconduct relating to a published article in their journal, the publisher or editor shall follow COPE's guidelines (or equivalent) in dealing with allegations. | |
| Alternative oxidase | AOX | Alternative quinol oxidases AOX are membrane-bound enzymes capable of supporting cyanide- and antimycin A-resistant mitochondrial respiration. AOX catalyzes the oxidation of ubiquinol and the reduction of oxygen to water in a four-electron process. As this bypasses several proton-translocating steps, induction of this alternative pathway is associated with a reduction of ATP production per oxygen consumed. AOX is found in most plants (including microalgae), many fungi and protists, but is not expressed in animals. AOX is inhibited by salicylhydroxamic acid (SHAM). Expression and activity of the enzyme are modified by environmental conditions such as temperature, oxidative stress, nutrient availability, and pathogens such as viruses. |
| Ambiguity crisis | The ambiguity crisis is a contemporary crisis comparable to the credibility or reproducibility crisis in the biomedical sciences. The term 'crisis' is rooted etymologically in the Greek word krinein: meaning to 'separate, decide, judge'. In this sense, science and communication in general are a continuous crisis at the edge of separating clarity or certainty from confusing double meaning, or obscure 'alchemical' gibberish, or even fake-news. Reproducibility relates to the condition of repeating and confirming calculations or experiments presented in a published resource. While ambiguity is linked to relevant issues of reproducibility, it extends to the communications space of terminological and graphical representations of concepts. Type 1 ambiguities are the inevitable consequence of conceptual evolution, in the process of which ambiguities are replaced by experimentally and theoretically supported paradigm shifts to clear-cut theorems. In contrast, type 2 ambiguities are traced in publications that reflect merely a disregard and ignorance of established concepts without an attempt to justify the inherent deviations from high-quality science. There are many shades of grey between these types of ambiguity. | |
| Ammonia solution concentrated | NH3 | Concentrated ammonia solution (25 % - 30 % ammonium hydroxide solution, ammonia) is used for the service of the polarographic oxygen sensor OroboPOS. After opening the commercial solution, the concentration of ammonia may decline during storage and may render the ammonia stock ineffective for sensor service. Source: A commercially available solution from a drugstore is sufficient for this cleaning purpose |
| Amount of substance | n [mol] | The amount of substance n is a base physical quantity, and the corresponding SI unit is the mole [mol]. Amount of substance (sometimes abbreviated as 'amount' or 'chemical amount') is proportional to the number NX of specified elementary entities X, and the universal proportionality constant is the reciprocal value of the Avogadro constant (SI), nX = NX·NA-1 nX contained in a system can change due to internal and external transformations, dnX = dinX + denX In the absence of nuclear reactions, the amount of any atom is conserved, e.g., for carbon dinC = 0. This is different for chemical substances or ionic species which are produced or consumed during the advancement of a reaction r, A change in the amount of Xi, dni, in an open system is due to both the internal formation in chemical transformations, drni, and the external transfer, deni, across the system boundaries. dni is positive if Xi is formed as a product of the reaction within the system. deni is negative if Xi flows out of the system and appears as a product in the surroundings (Cohen 2008 IUPAC Green Book). |
| Amp calibration - DatLab | F5 | Amp calibration indicates the calibration of the amperometric O2k-channel. |
| Ampere | A | The ampere, symbol A, is the SI unit of electric current. It is defined by taking the fixed numerical value of the elementary charge e to be 1.602 176 634 × 10−19 when expressed in the unit C, which is equal to A s, where the second is defined in terms of ΔνCs. |
| Amperometric,Amp | F7 | After selection of the Amperometric, Amp channel in the O2k configuration, an Amperometric, Amp tab will appear in the O2k control [F7] window. Set the desired light intensity (0-1600) in the field ´Fluo intensity´ and the desired amplification of the signal (1-1000) in the field ´Gain for Fluo sensor´in the Amperometric, Amp window followed by a left-click Send to O2k. Switching off the illumination before each fluorometric measurement is routinely required. |
| Amplex UltraRed | AmR | Amplex® UltraRed (AmR) is used as an extrinsic fluorophore for measurement of hydrogen peroxide production (ROS) by cells or mitochondrial preparations. The reaction of H2O2 and AmR is catalyzed by horseradish peroxidase to produce the red fluorescent compound resorufin (excitation wavelength 563 nm, emission 587 nm; the fluorescent product according to the supplier is called UltroxRed in the case of Amplex® UltraRed which has a similar structure to resorufin). The change of emitted fluorescence intensity is directly proportional to the concentration of H2O2 added, whereby the H2O2 is consumed. |
| Amplitude | The amplitude of the absorbance spectrum can be described in terms of the absorbance differences between the characteristic peaks (absorbance maxima) and troughs (absorbance minima) (see absorbance spectrum) for substances present in the sample. | |
| Amytal | Amy | Amytal sodium salt (synonym: amobarbital; 5-Ethyl-5-isoamylbarbituric acid) is a barbiturate drug and an inhibitor of Complex I. |
| Anaerobic | Anaerobic metabolism takes place without the use of molecular oxygen, in contrast to aerobic metabolism. The capacity for energy assimilation and growth under anoxic conditions is the ultimate criterion for facultative anaerobiosis. Anaerobic metabolism may proceed not only under anoxic conditions or states, but also under hyperoxic and normoxic conditions (aerobic glycolysis), and under hypoxic and microxic conditions below the limiting oxygen pressure. | |
| Anaplerosis | Anaplerosis is the process of formation of intermediates of the tricarboxylic acid cycle. Malic enzyme (mtME), phosphoenolpyruvate carboxykinase (PEPCK), propionyl-CoA carboxylase, pyruvate carboxylase and proline dehydrogenase play important roles in anaplerosis. | |
| Anaplerotic pathway control state | a | Anaplerotic pathway control states are fuelled by single substrates which are transported into the mitochondrial matrix and increase the pool of intermediates of the tricarboxylic acid cycle. Malic enzyme (mtME), phosphoenopyruvate carboxykinase (PEPCK), propionyl-CoA carboxylase, and pyruvate carboxylase play important roles in anaplerosis. The glutamate-anaplerotic pathway control state and malate-anaplerotic pathway control state are the most important anaplerotic substrate control states (aN). |
| Anoxia | anox | Ideally the terms anoxia and anoxic (anox, without oxygen) should be restricted to conditions where molecular oxygen is strictly absent. Practically, effective anoxia is obtained when a further decrease of experimental oxygen levels does not elicit any physiological or biochemical response. The practical definition, therefore, depends on (i) the techiques applied for oxygen removal and minimizing oxygen diffusion into the experimental system, (ii) the sensitivity and limit of detection of analytical methods of measuring oxygen (O2 concentration in the nM range), and (iii) the types of diagnostic tests applied to evaluate effects of trace amounts of oxygen on physiological and biochemical processes. The difficulties involved in defining an absolute limit between anoxic and microxic conditions are best illustrated by a logarithmic scale of oxygen pressure or oxygen concentration. In the anoxic state (State 5), any aerobic type of metabolism cannot take place, whereas anaerobic metabolism may proceed under oxic or anoxic conditions. |
| Antimycin A | Ama | Antimycin A is an inhibitor of Complex III (CIII). It binds to the Qi site of CIII and inhibits the transfer of electrons from heme bH to oxidized Q (Qi site inhibitor). High concentrations of antimycin A also inhibit acyl-CoA oxidase and D-amino acid oxidase. |
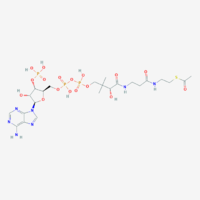
| Ap5A | Ap5A | P1,P5-Di(adenosine-5')pentaphosphate (Ap5A) is an inhibitor of adenylate kinase (ADK), the enzyme which rephosphorylates AMP to ADP, consuming ATP (ATP + AMP ↔ 2 ADP). |
| Aqua destillata | a.d. | Aqua destillata (a.d.) is the Latin name for distilled water, H2O. When a.d. is used in various solution protocols, it may indicate that water with the highest possible quality or lowest possible level of impurities should be used, as may be reached not only with distilled water but also with high-purity deionised water. |
| ArXiv preprint server | arXiv | arXiv is a classic preprint server initiated in 1991 by Paul Ginsparg. {Quote} arXiv.org is a highly-automated electronic archive and distribution server for research articles. Covered areas include physics, mathematics, computer science, nonlinear sciences, quantitative biology, quantitative finance, statistics, electrical engineering and systems science, and economics. arXiv is maintained and operated by Cornell University with guidance from the arXiv Scientific Advisory Board and the arXiv Member Advisory Board, and with the help of numerous subject moderators. {end of Quote}. arXiv rejects abstracts that are submitted without accompanying paper. |
| Artemisinin | Artemisinin and various derivatives are potent anti-malaria drugs which have additionally anti-tumorigenic effects, particularly when targeted at mitochondria. The anti-malaria effect is associated with artemisinin's action on heme. Mitochondria are involved in the synthesis of heme, and may play additional roles in the anti-tumorigenic effect of artemisinin. | |
| Ascorbate | As | In respiratory assays for cytochrome c oxidase activity (Complex IV, CIV), ascorbate is added as regenerating system to maintain TMPD in a reduced state. It has to be titrated into the respiration medium prior to the addition of TMPD, otherwise the autoxidation reaction velocity is permanently elevated. |
| Asia Society for Mitochondrial Research and Medicine | ASMRM | The Asia Society for Mitochondrial Research and Medicine (ASMRM) was founded in 2003 to share the latest knowledge on mitochondrial research. |
| Aspirin | Aspirin is a widely applied drug that requires dosage adjusted to individual body mass. It is a non-selective COX inhibitor and exerts an effect on long-chain fatty acid transport into mitochondria. | |
| Assay | An experimental assay is a method to obtain a measurement with a defined instrument on a sample or subsample. Multiple assay types may be applied on the same sample or subsample, if the measurement does not destroy it. For instance, the wet weight of a permeabilized muscle fibre preparation can be determined based on a specific laboratory protocol (gravimetric assay), maintaining the functional integrity of the sample, which then can be used in a respirometric assay, followed by a spectrophotometric assay for measurement of protein content. The experimental design determines which types of assays have to be applied for a complete experiment. Destructive assays, such as determination of protein content or dry weight, can be applied on a sample only after performing a respirometric assay, or on a separate subsample. The experimental variability is typically dominated by the assay with the lowest resolution or signal to noise ratio. The signal to noise ratio may be increased by increasing the number, n, of repetitions of measurements on subsamples. Evaluation of procedural variation ('experimental noise') due to instrumental resolution and handling requires subsampling from homogenous samples. | |
| Atractyloside | Atr | Atractyloside is an inhibitor of the adenine nucleotide translocator (ANT). It is an extremely toxic glycoside that inhibits oxidative phosphorylation by blocking the transfer of adenosine nucleotides through the mitochondrial membrane. |
| Attached cells | Many cell types are grown in culture as attached cells, such as endothelial or neuronal cells in a monolayer. | |
| Attribute | Attribute in general is a characteristic or property. In databases an attribute describes a column in a table. Rows then represent the according attribute values. | |
| Auranofin | AF | Auranofin (AF) is a gold complex which inhibites thioredoxin reductase (TrxR). |
| Automatic pan - DatLab | Automatic pan (only for real-time data recording) toggles automatic panning on/off by clicking in the O2k status line. If it is on (green), the time range is maintained while the time axis always shows the currently recorded data, i.e. the value of the offset (minimum value) increases as experimental time proceeds. If it is off (yellow), the time axis is static. This allows for manually panning backwards to observe previous sections of the experiment at a given time range. In this mode, the actual experimental time may be off-scale. Toggle between "Pan auto" and "Pan off" by a left-click on the text. It does not influence continuous data recording. It is recommended to maintain automatic panning on during the experiment, except for specifically viewing earlier sections of the experiment. | |
| Autoscale | Autoscale zooms in or out of the selected period with Autoscale time axis, Autoscale Y1 (Y2) axes and Automatic pan. | |
| Autoscale Y1 (Y2) axes | Autoscale Y1 (Y2) axes: Autoscaling the measured values (full data range) on the Y1 (Y2) axis in the selected plot. | |
| Autoscale time axis | Autoscale time axis gives an overview of the entire experimental period. | |
| Autoxidation | This definition is insufficient and needs elaboration. Autoxidation is a slow process implying oxidation of carbohydrates through oxygen in open air, leading to a primary formation of peroxides and hydroperoxides. UV radiation can speed up this process. | |
| Averaging | In order to improve the signal-to-noise ratio a number of sequential spectra may be averaged over time. The number of spectra to be averaged can be set prior to carrying out the measurements, or afterwards during data analysis. | |
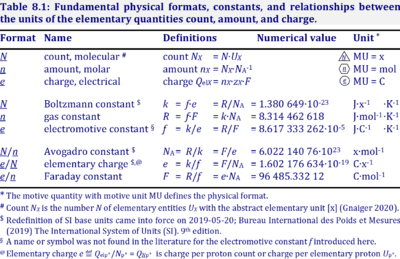
| Avogadro constant | NA [x·mol-1] | {Quote} The Avogadro constant NA is a proportionality constant between the quantity amount of substance (with unit mole) and the quantity for counting entities ... One mole contains exactly 6.022 140 76 × 1023 elementary entities. This number is the fixed numerical value of the Avogadro constant, NA, when expressed in the unit mol−1 and is called the Avogadro number {End of Quote: Bureau International des Poids et Mesures 2019 The International System of Units (SI)}. Thus the Avogadro constant NA has the SI unit 'per mole' [mol-1], but more strictly the unit for counting entities per amount is 'units per mole' [x·mol-1] (compare elementary charge). Therefore, NA is 'count per amount' with units 'counting units per mole'. The Avogadro constant times elementary charge is the Faraday constant. |
| Azide | Azd | Sodium azide is an inhibitor of Complex IV/cytochrome c oxidase (CIV, COX, CcO). |
| BAM15 | BAM15 | 2-fluorophenyl){6-[(2-fluorophenyl)amino](1,2,5-oxadiazolo[3,4-e]pyrazin-5-yl)}amine (BAM15) is a protonophore or uncoupler of oxidative phosphorylation detected in a screen for uncoupling agents exerting less toxicity than commonly used uncouplers and first described by Kennwood et al. 2013. In their comparison of BAM15 with FCCP it was shown to increase oxygen flux to a similar extent as the classical uncoupler, to display a much broader range of concentrations inducing maximum respiration, to stimulate no formation of H2O2, to leave cellular membrane potential unaffected, and to ultimately exert less cytotoxicity. |
| BME cutoff points | BME cutoff | Obesity is defined as a disease associated with an excess of body fat with respect to a healthy reference condition. Cutoff points for body mass excess, BME cutoff points, define the critical values for underweight (-0.1 and -0.2), overweight (0.2), and various degrees of obesity (0.4, 0.6, 0.8, and above). BME cutoffs are calibrated by crossover-points of BME with established BMI cutoffs. |
| Background state | Y | The background state Y (background rate YX) is the non-activated or inhibited respiratory state at background rate, which is low in relation to the higher rate ZX in the reference state Z. The transition from the background state to the reference state is a step change. A metabolic control variable X (substrate, activator) is added to the background state to stimulate flux to the level of the reference state. Alternatively, the metabolic control variable X is an inhibitor, which is present in the background state Y, but absent in the reference state Z. The background state is the baseline of a single step in the definition of the flux control efficiency. In a sequence of step changes, the common baseline state is the state of lowest flux in relation to all steps, which can be used as a baseline correction. |
| Balance | In transmission spectrophotometry blank cuvettes are used to record the incident light intensity (I0) prior to absorbance measurements. (See white balance for reflectance spectrophotometry, remittance spectrophotometry). | |
| Bandwidth | Bandwidth is measured in nanometers in terms of the full width half maximum of a peak. This is the portion of the peak that is greater than half of the maximum intensity of that peak. | |
| Barometric pressure | pb [Pa] | Barometric pressure, pb, is an important variable measured for calibration of oxygen sensors in solutions equilibrated with air. The atm-standard pressure (1 atm = 760 mmHg = 101.325 kPa) has been replaced by the SI standard pressure of 100 kPa. The partial pressure of oxygen, pO2, in air is a function of barometric pressure, which changes with altitude and locally with weather conditions. The partial oxygen pressure declines by 12 % to 14 % per 1,000 m up to 6,000 m altitude, and by 15 % to 17 % per 1,000 m between 6,000 and 9,000 m altitude. The O2k-Barometric Pressure Transducer is built into the Oroboros O2k as a basis for accurate air calibrations in high-resolution respirometry. For highest-level accuracy of calculation of oxygen pressure, it is recommended to compare at regular intervals the barometric pressure recording provided by the O2k with a calibrated barometric pressure recording at an identical time point and identical altitude. The concept of gas pressure or barometric pressure can be related to the generalized concept of isomorphic pressure. |
| Barth Syndome | BTHS | Barth Syndome (BTHS) is an X-linked genetic condition that is caused by a mutation in the tafazzin gene (taz). This mutation causes cardiolipin abnormalities, cardiomyopathy, neutropenia, muscle weakness, growth delay, and exercise intolerance. Contributed by Sparagna GC 2016-04-24 |
| Basal respiration | BMR | Basal respiration or basal metabolic rate (BMR) is the minimal rate of metabolism required to support basic body functions, essential for maintenance only. BMR (in humans) is measured at rest 12 to 14 hours after eating in a physically and mentally relaxed state at thermally neutral room temperature. Maintenance energy requirements include mainly the metabolic costs of protein turnover and ion homeostasis. In many aerobic organisms, and particularly well studied in mammals, BMR is fully aerobic, i.e. direct calorimetry (measurement of heat dissipation) and indirect calorimetry (measurement of oxygen consumption multiplied by the oxycaloric equivalent) agree within errors of measurement (Blaxter KL 1962. The energy metabolism of ruminants. Hutchinson, London: 332 pp [1]). In many cultured mammalian cells, aerobic glycolysis contributes to total ATP turnover (Gnaiger and Kemp 1990 [2]), and under these conditions, 'respiration' is not equivalent to 'metabolic rate'. Basal respiration in humans and skeletal muscle mitochondrial function (oxygen kinetics) are correlated (Larsen et al 2011 [3]). » MiPNet article |
| Base quantities and count | Template:Base quantities and count | |
| Baseline state | The baseline state in a sequence of step changes is the state of lowest flux in relation to all steps, which can be used as a baseline correction. Correction for residual oxygen consumption, ROX, is an example where ROX is the baseline state. In a single step, the baseline state is equivalent to the background state. | |
| Beer-Lambert law | B-L law | This law states that the transmittance (T) of light though a sample is given by: T = e-εbc, where ε is the molar extinction coefficient, b is the pathlength of the light through the cuvette (in mm) and c is the concentration of the pigment in the sample (in mM). Transforming this equation, it can be seen that the absorbance of light (A) is simply given by A = εbc. |
| Beryllium sulfate | BeS | Beryllium sulfate is used in combination with sodium fluoride to form beryllium trifluoride (BeF3−), to inhibit the ATP synthase if it is exposed by disruption of the mitochondrial membranes. |
| Bias | The bias is defined as the difference between the mean of the measurements and the reference value. In general, the measuring instrument calibration procedures should focus on establishing and correcting it. | |
| BioRxiv preprint server for biology | bioRxiv | bioRxiv (pronounced "bio-archive") is a free online archive and distribution service for unpublished preprints in the life sciences. It was launched in 2013 by Cold Spring Harbor Laboratory Press in New York, and is operated by Cold Spring Harbor Laboratory, a not-for-profit research and educational institution. By posting preprints on bioRxiv, authors are able to make their findings immediately available to the scientific community and receive feedback on draft manuscripts before they are submitted to journals. bioRxiv is intended for rapid sharing of new research. Some review articles contain new data/analyses and may therefore be deemed appropriate. Reviews that solely summarize existing knowledge are not appropriate and neither are term papers, book excerpts, and undergraduate dissertations. |
| Bioblast | ||
| Bioblast alert 2020 | ||
| Bioblast alert 2021 | ||
| Bioblast alert 2022 | ||
| Bioblast alert 2023 | ||
| Bioblast alert 2024 | ||
| Bioblast track |