Semantic search
| Term | Abbreviation | Description |
|---|---|---|
| De Onis 2007 Bull World Health Organization | ||
| Dead cells | dce | Dead cells dce are characterized by the loss of plasma membrane barrier function. The total cell count (Nce) is the sum of viable cells (Nvce) and dead cells (Ndce). |
| Decimal marker and spaces between groups of numerals | . | A decimal marker is used to separate the integral part of numbers from the decimal part. The SI recommends: "the symbol for the decimal marker shall be either the point on the line or the comma on the line". In English language versions, the dot (point on the line) should be used uniquely as the decimal marker. To avoid ambiguities, BEC follows the SI recommendation that “Numbers may be divided in groups of three in order to facilitate reading; neither dots nor commas are ever inserted in the spaces between groups” (pages 183-184). |
| Default label | The Default label is the system default value for the axis label. These labels are changed automatically, according to the selected channel and unit. To change this label enter a Custom label. | |
| Delete points | Select Delete points in the Mark information window to remove all data points in the marked section of the active plot. See also Interpolate points and Restore points or Recalculate slope. | |
| Density | ρ, C, D | Density, mass density ρ = m·V-1 [kg·m-3], is mass m divided by volume V. Surface density ρA = m·A-1 [kg·m-2] (SI). For a pure sample S, the mass density ρS = mS·VS-1 [kg·m-3] is the mass m of pure sample S per volume VS of the pure sample. With density ρ thus defined, the 'amount density' of substance B is ρB = nB·VB-1 [mol·m-3]. This is not a commonly used expression, but the inverse is defined as the molar volume of a pure substance (IUPAC), Vm,B = VB·nB-1 [m3·mol-1]. The pure sample is a pure gas, pure liquid or pure solid of a defined elementary entity. The amount concentration, cB = nB·V-1 [mol·m-3] is the amount nB of substance B divided by the volume V of the mixture (IUPAC), and this is not called an 'amount density'. The term 'amount density' is reserved for an amount of substance per volume VS of the pure substance. This explicit distinction between 'density' related to the volume of the sample and 'concentration' related to the total volume of the mixture is very helpful to avoid confusion. Further clarification is required in cases, when the mass density ρs of the sample in the mixture differs from the mass density ρS of the pure sample before mixing. Think of a sample S of pure ethanol with a volume of 1 L at 25 °C, which is mixed with a volume of 1 L of pure water at 25 °C: after the temperature of the mixture has equilibrated to 25 °C, the total volume of the mixture is less than 2 L, such that the volume VS of 1 L pure ethanol has diminished to a smaller volume Vs of ethanol in the mixture; the density of ethanol in the mixture is higher than the density of pure ethanol (this is incomplete additivity). The volume Vs of sample s in a mixture is by definition smaller than the total volume V of the mixture. Sample volume VS and system volume V are identical, but this applies only to the case of a pure sample. Concentration is related to samples s per total volume V of the mixture, whereas density is related to samples S or s per volume VS = V or Vs < V, respectively (BEC 2020.1). |
| Derivative spectroscopy | Derivative spectroscopy can be used to eliminate interfering artefacts or species. A first order derivative will remove a constant background absorbance across the spectral range. A second order derivative spectrum will remove a species whose absorbance is linearly dependent upon the wavelength, etc.. | |
| Deselect channels | F7 | Channels can be selected/deselected in DatLab in the O2k configuration. Deselect all O2k-MultiSensor channels in O2k-Core applications. Select only the specifically used channels in O2k-MultiSensor applications. |
| Detector | A detector is a device that converts the light falling upon it into a current or voltage that is proportional to the light intensity. The most common devices in current use for fluorometry and spectrophotometry are photodiodes and photodiode arrays. | |
| Diapause | Diapause is a preprogrammed form of developmental arrest that allows animals to survive harsh environmental conditions and may also allow populations to synchronize periods of growth and reproduction with periods of optimal temperatures and adequate water and food. Diapause is endogenously controlled, and this dormancy typically begins well before conditions become too harsh to support normal growth and development [1,2]. » MiPNet article | |
| Dicarboxylate carrier | DIC | The dicarboxylate carrier is a transporter which catalyses the electroneutral exchange of malate2- (or succinate2-) for inorganic phosphate, HPO42-. |
| Difference spectrum | A difference spectrum is an absorbance spectrum obtained by subtracting that of one substance from that of another. For example, a difference spectrum may be plotted of the absorbance spectrum obtain ed from reduced cytochrome c and subtracting the absorbance spectrum from the same concentration of cytochrome c in its oxidised state. The difference spectrum may be used to quantify the amount to which the cytochrome c is reduced. This can be achieved with the aid of a reference spectrum (or spectra) and the least squares method. | |
| Different O2 fluxes in left and right chamber | What are potential causes for different O2 fluxes in the left and right chamber? | |
| Diffraction gratings | Diffraction gratings are dispersion devices that are made from glass etched with fine grooves, spaced at the same order of magnitude as the wavelength of the light to be dispersed, and then coated with aluminium to reflect the light to the photodiode array. Diffraction gratings reflect the light in different orders and filters need to be incorporated to prevent overlapping. | |
| Digital Object Identifier | DOI | A Digital Object Identifier, DOI, is a persistent identifier used to uniquely identify online publications in order to ensure they remain traceable, searchable and citable over the long term. Compared to other types of persistent identifiers, the DOI system is widespread and well established in the life sciences arena, and it provides widely accepted visible proof that a publication is citable. |
| Digitonin | Dig | Digitonin is a mild detergent that permeabilizes plasma membranes selectively due to their high cholesterol content, whereas mt-membranes with lower cholesterol content are affected only at higher concentrations. Digitonin is a natural product and thus the effective concentration has to be determined by titrations for every batch. The optimum effective digitonin concentrations for complete plasma membrane permeabilization of cultured cells can be determined directly in a respirometric protocol (see: SUIT-010 O2 ce-pce D008). |
| Dihydro-orotate dehydrogenase | DhoDH | Dihydro-orotate dehydrogenase is an electron transfer complex of the inner mitochondrial membrane, converting dihydro-orotate (Dho) into orotate, and linking electron transfer through the Q-junction to pyrimidine synthesis and thus to the control of biogenesis. |
| Dihydroethidium | DHE | Dihydroethidium (also called hydroethidine) is a cell permeant fluorescent probe used to analyse superoxide presence. It is a reduced form of ethidium that presents blue fluorescence, and after oxidation by superoxide becomes able to intercalate DNA and emits red fluorescence (excitation wavelength ~518–535 nm, emission ~605–610 nm). It has been used to detect superoxide by HPLC and by fluorescence microscopy. |
| Dilution effect | Dilution of the concentration of a compound or sample in the experimental chamber by a titration of another solution into the chamber. | |
| Dimension | Dimensions are defined in the SI {Quote}: Physical quantities can be organized in a system of dimensions, where the system used is decided by convention. Each of the seven base quantities used in the SI is regarded as having its own dimension. .. All other quantities, with the exception of counts, are derived quantities, which may be written in terms of base quantities according to the equations of physics. The dimensions of the derived quantities are written as products of powers of the dimensions of the base quantities using the equations that relate the derived quantities to the base quantities. There are quantities Q for which the defining equation is such that all of the dimensional exponents in the equation for the dimension of Q are zero. This is true in particular for any quantity that is defined as the ratio of two quantities of the same kind. .. There are also some quantities that cannot be described in terms of the seven base quantities of the SI, but have the nature of a count. Examples are a number of molecules, a number of cellular or biomolecular entities (for example copies of a particular nucleic acid sequence), or degeneracy in quantum mechanics. Counting quantities are also quantities with the associated unit one. {end of Quote: p 136, Bureau International des Poids et Mesures 2019 The International System of Units (SI)} | |
| Dimethyl sulfoxide | DMSO | Dimethyl sulfoxide is a polar aprotic solvent that dissolves both polar and nonpolar compounds and is miscible in a wide range of organic solvents as well as water. DMSO may also be used as a cryoprotectant, added to cell media to reduce ice formation and thereby prevent cell death during the freezing process. |
| Dinitrochlorobenzene | DNCB | Dinitrochlorobenzene (1-chloro-2,4-dinitrobenzene) (DNCB) is a glutathione (GSH) inhibitor. |
| Dinitrophenole | DNP | 2,4-dinitrophenole (C6H4N2O5; M = 184.11 g·mol-1) is a protonophore acting as an uncoupler of oxidative phosphorylation. |
| Directive | A directive is a legal act of the European Union, which requires member states to achieve a particular result without dictating the means of achieving that result. | |
| Directory of Open Access Journals | DOAJ | The Directory of Open Access Journals is a free online directory that indexes and provides access to open access peer-reviewed journals. |
| Disconnect - DatLab | Ctrl+Shift+D | Disconnect DatLab 8 from the O2k. This option is not available while recording a measurement (Stop measurement first). DatLab 7 : Save and Disconnect |
| Discontinuous system | In a discontinuous system, gradients in continuous systems across the length, l, of the diffusion path [m], are replaced by differences across compartmental boundaries of zero thickness, and the local concentration is replaced by the free activity, α [mol·dm-3]. The length of the diffusion path may not be constant along all diffusion pathways, spacial direction varies (e.g., in a spherical particle surrounded by a semipermeable membrane), and information on the diffusion paths may even be not known in a discontinuous system. In this case (e.g., in most treatments of the protonmotive force) the diffusion path is moved from the (ergodynamic) isomorphic force term to the (kinetic) mobility term. The synonym of a discontinuous system is compartmental or discretized system. In the first part of the definition of discontinuous systems, three compartments are considered: (1) the source compartment A, (2) the sink compartment B, and (3) the internal barrier compartment with thickness l. In a two-compartmental description, a system boundary is defined of zero thickness, such that the barrier comparment (e.g., a semipermeable membrane) is either part of the system (internal) or part of the environment (external). Similarly, the intermediary steps in a chemical reaction may be explicitely considered in an ergodnamic multi-comparment system; alternatively, the kinetic analysis of all intermediary steps may be collectively considered in the catalytic reaction mobility, reducing the measurement to a two-compartmental analysis of the substrate and product compartments. | |
| Dispersion devices | A dispersion device diffracts light at different angles according to its wavelength. Traditionally, prisms and diffraction gratings are used, the latter now being the most commonly used device in a spectrofluorometer or spectrophotometer. | |
| Display DatLab help | Display DatLab help In this section, we present some issues that could happen during your data analysis related to the graphs display and how to fix them quickly. Case in which an issue might occur:
In the event of a frozen display of the graphs, try the alternatives below:
| |
| Display Power-O2k | The Power-O2k number, which is set in the pull-down menu Oroboros O2k \ O2k configuration, is shown in the active graph. To show it in graphs copied to clipboard, the option "Show Oroboros icon in clipboard files" must be enabled in the Graph-menu Graph options - DatLab. | |
| Display numerical value | If Display numerical value the current numerical values are displayed in the graph for the active plots on the Y1 axis and Y2 axis (during data acquisition only). | |
| Dithionite | Dit Dit - (The abbreviation 'Dith' has been used previously and is stepwise replaced by Dit.) | The sodium salt of Dithionite Na2S2O4 (Dit) is the 'zero oxygen solution powder' used for calibration of oxygen sensors at zero oxygen concentration, or for stepwise reduction of oxygen concentrations in instrumental O2 background tests. It is not recommended to use dithionite in experiments with biological samples or several multisensor approaches, for these see Setting the oxygen concentration. |
| Drift | The most common cause of drift is variation in the intensity of the light source. The effect of this can be minimised by carrying out a balance at frequent intervals. | |
| Dual wavelength analysis | If a sample contains a number of absorbing substances, it is sometimes possible to select discrete pairs of wavelengths at which the change in absorbance of a particular substance (due to oxidation or reduction, for example) is largely independent of changes in the absorbance of other substances present. Dual wavelength analysis can be carried out for cytochrome c by subtracting the absorbance at 540 nm from that at 550nm in order to give a measure of the degree of reduction. Similarly, by subtracting the absorbance at 465 nm from that at 444 nm, an indicator of the redox state of cytochrome aa3 can be obtained. | |
| Duroquinol | DQ | ET-pathway level 2 is supported by duroquinol DQ feeding electrons into Complex III (CIII) with further electron transfer to CIV and oxygen. Upstream pathways are inhibited by rotenone and malonic acid in the absence of other substrates linked to ET-pathways with entry into the Q-junction. |
| Dyscoupled respiration | Dyscoupled respiration is LEAK respiration distinguished from intrinsically (physiologically) uncoupled and from extrinsic experimentally uncoupled respiration as an indication of extrinsic uncoupling (pathological, toxicological, pharmacological by agents that are not specifically applied to induce uncoupling, but are tested for their potential dyscoupling effect). Dyscoupling indicates a mitochondrial dysfunction. In addition to intrinsic uncoupling, dyscoupling occurs under pathological and toxicological conditions. Thus a distinction is made between physiological uncoupling and pathologically defective dyscoupling in mitochondrial respiration. | |
| E | e, E | » elementary charge e = 1.602 176 634∙10-19 C∙x-1 » Euler's number e ~ 2.718 281 828 459 » ET capacity E |
| E-L coupling efficiency | jE-L | |
| E-L net ET capacity | E-L | |
| E-P control efficiency | jE-P | |
| E-P excess capacity | E-P | |
| E-R control efficiency | jE-R | |
| E-R reserve capacity | E-R | |
| ET capacity | E | |
| ET-pathway competent state | Electron transfer pathway competent state, see Electron-transfer-pathway state. | |
| ET-pathway substrate types | n.a. | See Electron-transfer-pathway state |
| EUROMIT |
EUROMIT is a group based in Europe for organizing International Meetings on Mitochondrial Pathology. | |
| Ectotherms | Ectotherms are organisms whose body temperatures conform to the thermal environment. In many cases, therefore, ectotherms are poicilothermic. | |
| Editorial board participation | Editorial board participation is a topic addressed in COPE core practices for research. | |
| Elamipretide | Bendavia | Bendavia (Elamipretide) was developed as a mitochondria-targeted drug against degenerative diseases, including cardiac ischemia-reperfusion injury. Clinical trials showed variable results. It is a cationic tetrapeptide which readily passes cell membranes, associates with cardiolipin in the mitochondrial inner membrane. Supercomplex-associated CIV activity significantly improved in response to elamipretide treatment in the failing human heart. |
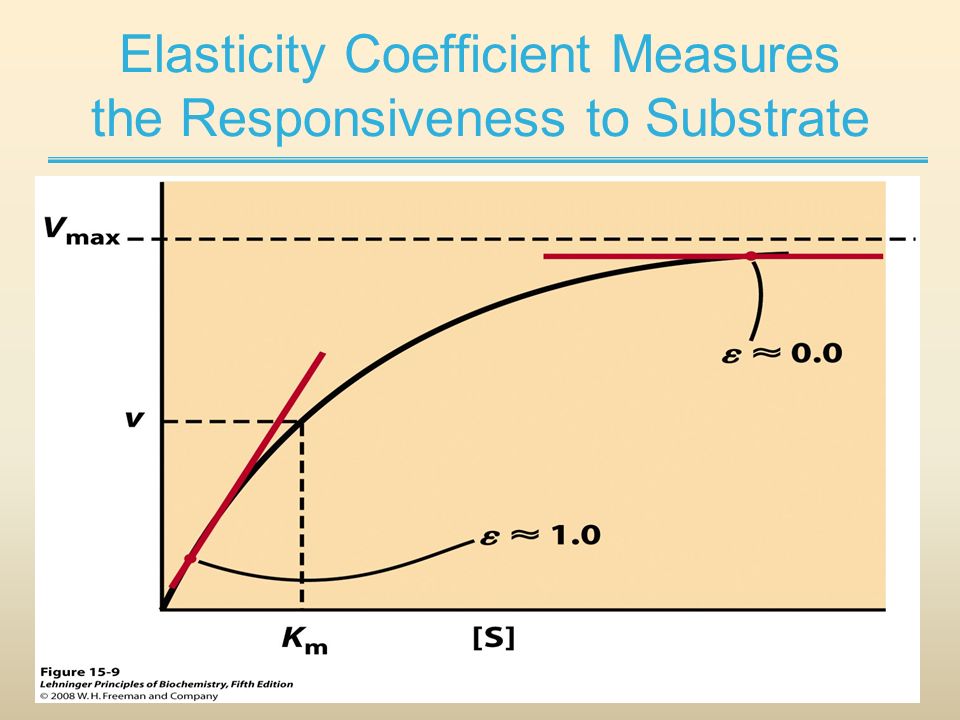
| Elasticity | ε | According to David Fell, "Elasticities are properties of individual enzymes and not the metabolic system. The elasticity of an enzyme to a metabolite is related to the slope of the curve of the enzyme's rate plotted against metabolite concentration, taken at the metabolite concentrations found in the pathway in the metabolic state of interest. It can be obtained directly as the slope of the logarithm of the rate plotted against the logarithm of the metabolic concentration. The elasticity will change at each point of the curve (s,v) and must be calculated for the specific concentration of the metabolite (s) that will give a specific rate (r) of the enzyme activity" (See Figure). |
| Electric current | Iel [A = C·s-1]; [mol·s-1]; [x·s-1] | Current or electric flow Iel is the advancement of charge per unit of time, expressed in the SI base unit ampere [C·s-1 = A]. Electrons or ions are the current-carrying motive entities of electric flow. Electrons e- are negatively charged subatomic particles carrying 'negative electricity' with a mass that is about 1/1700 of the smallest particle — the proton — carrying 'positive electricity' (Thompson 1906). Correspondingly the velocity of electrons is much higher than that of protons or any other (larger) ion. Current is the velocity v of paticles times the number of motive charges. Therefore, electron current Ie- is of a different nature from electric current Ielχ carried by all species i of ions Xi (cations and anions) summarized as χ = Σ(zi·Xi). Whereas Ie- is the net translocation of electrons moving forwards and backwards, Ielχ is the net translocation of charges carried by different cations and anions. In contrast, ion current IelX of a specific ion X is the partial translocation of charges carried by net translocation of ion X only. If cation current IelX+ is antagonized entirely by counterion current IelY- as the process of antiport, then the electric current Ielχ is zero. The (net) electric current in a compartmental system is driven by the electric force ΔelFp+ or electric potential difference ΔΨp+, whereas a compensated ion/counterion antiport current is insensitive to the electric potential difference. |
| Electric current density | j [C·m-2] | Electric current density is current divided by area, j=I·A-1 [C·m-2]. Compare: density. |
| Electrochemical constant | f [J·C-1·K-1] | The electrochemical constant f has the SI unit for energy per charge per temperature [J·C-1·K-1]. f = k·e-1, the Boltzmann constant k divided by the elementary charge e. f = R·F-1, the gas constant R divided by the Faraday constant F. |
| Electrolyte\Reference-Electrode | Electrolyte\Reference-Electrode for Reference-Electrode\2.4 mm | |
| Electron flow | Ie | Electron flow through the mitochondrial Electron transfer pathway (ET-pahway) is the scalar component of chemical reactions in oxidative phosphorylation (OXPHOS). Electron flow is most conveniently measured as oxygen consumption (oxygraphic measurement of oxygen flow), with four electrons being taken up when oxygen (O2) is reduced to water. |
| Electron leak | Electrons that escape the electron transfer pathway without completing the reduction of oxygen to water at cytochrome c oxidase, causing the production of ROS. The rate of electron leak depends on the topology of the complex, the redox state of the moiety responsible of electron leakiness and usually on the protonmotive force (Δp). In some cases, the Δp dependance relies more on the ∆pH component than in the ∆Ψ. | |
| Electron transfer pathway | ET pathway | In the mitochondrial electron transfer pathway (ET pathway) electrons are transferred from externally supplied reduced fuel substrates to oxygen. Based on this experimentally oriented definition (see ET capacity), the ET pathway consists of (1) the membrane-bound ET pathway with respiratory complexes located in the inner mt-membrane, (2) TCA cycle and other mt-matrix dehydrogenases generating NADH and succinate, and (3) the carriers involved in metabolite transport across the mt-membranes. » MiPNet article |
| Electron-transfer-pathway state | ET-pathway state |
Electron-transfer-pathway states are obtained in mitochondrial preparations (isolated mitochondria, permeabilized cells, permeabilized tissues, tissue homogenate) by depletion of endogenous substrates and addition to the mitochondrial respiration medium of fuel substrates (CHNO) activating specific mitochondrial pathways, and possibly inhibitors of specific pathways. Mitochondrial electron-transfer-pathway states have to be defined complementary to mitochondrial coupling-control states. Coupling-control states require ET-pathway competent states, including oxygen supply. Categories of SUIT protocols are defined according to mitochondrial ET-pathway states. » MiPNet article |
| Electron-transferring flavoprotein Complex | CETF | Electron-transferring flavoprotein Complex (CETF) is a respiratory Complex localized at the matrix face of the inner mitochondrial membrane, supplies electrons to Q, and is thus an enzyme Complex of the mitochondrial Electron transfer pathway (ET-pathway). CETF links the ß-oxidation cycle with the membrane-bound electron transfer system in fatty acid oxidation (FAO). |
| Electronic-TIP2k Upgrading\O2k-Main Unit Series A-D | Electronic-TIP2k Upgrading\O2k-Main Unit Series A-D - Former Product : not required for O2k-Core, the O2k-Main Unit has to be returned to the OROBOROS workshop. | |
| Electronic-TIP2k Upgrading\O2k-Main Unit Series E | Electronic-TIP2k Upgrading\O2k-Main Unit Series E - Former Series : not required for O2k-Core, free of charge for Series E in conjunction with the purchase of the TIP2k-Module, the O2k-Main Unit has to be returned to the OROBOROS workshop. | |
| Elementary charge | e [C·x-1] | The elementary charge or proton charge e has the SI unit coulomb [C], but more strictly coulomb per elementary unit [C·x-1]. -e is the charge per electron. Elementary charge e is the charge per elementary entity H+ with SI unit [C] but canonical SI unit [C·x-1]. When the charge Qel [C] of a number Ne [x] of electrons e is divided by the count Ne, then the particle charge QNX (QNX) charge per elementary entity is obtained, -e = Qel/Ne [C·x-1]. e is also used as an atomic unit. |
| Elementary entity | UX [x] |
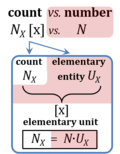
An elementary entity is an entity of type X, distinguished as a single unit of countable objects (X = molecules, cells, organisms, particles, parties, items) or events (X = beats, collisions, emissions, decays, celestial cycles, instances, occurrences, parties). "An elementary entity may be an atom, a molecule, an ion, an electron, any other particle or specified group of particles" (Bureau International des Poids et Mesures 2019). An elementary entity, therefore, needs to be distinguished from non-countable entities and the general class of entities X. This distinction is emphasized by the term 'elementary' (synonymous with 'elementary entity') with symbol UX and elementary unit [x]. If an object is defined as an assembly of particles (a party of two, a molecule as the assembly of a stoichiometric number of atoms), then the elementary is the assembly but not the assembled particle. A number of defined elementaries UX is a count, NX = N·UX [x], where N is a number, and as such N is dimensionless, and N is a number (stop) and is not 'a number of ..'. Elementaries are added as items to a count. The elementary UX has the dimension U of the count NX. The elementary UX has the same unit [x] as the count NX, or more accurately it gives the count the defining 'counting-unit', which is the 'elementary unit' [x]. From the definition of count as the number (N) of elementaries (U) of entity type X, it follows that count divided by elementary is a pure number, N = NX·UX-1. The unit x of a count can neither be the entity X nor a number. The elementary of type X defines the identity X of the elementary UX with the unit 'elementary unit' with symbol [x]. Since a count NX is the number of elementary entities, the elementary UX is not a count (UX is not identical with N·UX). |
| Elementary unit | x | The elementary unit [x] is the unit of a count NX [x]. The International System of Units defines the unit of a count as 1. Then the Number 1 is the Unit of the Count of Entities — NUCE. This causes a number of formal inconsistencies which are resolved by introducing the elementary unit [x] as the abstracted unit of Euclid’s unit, which is an elementary entity UX [x], and as the unit of Euclid’s number, which is a count NX [x]. |
| Enable DL-Protocol editing | Enable DL-Protocol editing is a novel function of DatLab 7.4 offering a new feature in DL-Protocols: flexibility. Fixed sequences of events and marks can be changed (Skip/Added) in a SUIT protocol by the user. Moreover, the text, instructions, concentrations and titration volumes of injections in a specific DL-Protocol can be edited and saved as user-specific DL-Protocol [File]\Export\DL-Protocol User (*.DLPU). To enable it, under the 'Protocols' tab in the menu, select the option 'Enable DL-Protocol editing', and then select the plot in which the marks will be set (e.g., O2 flux per V). Select the 'Overview' window, where you will be able to edit events and marks names, definition/state, final concentration and titration volumes, as well as select a mark as 'multi' for multiple titration steps, skip a mark, or add a new event or mark. After saving, export a DL-Protocol User (DLPU) and load it before running the next experiments. If users of DatLab versions older than DatLab 7.4 wish to alter the nature of the chemicals used or the sequence of injections, we ask them to contact the O2k-Technical Support. For more information:
| |
| Endergonic | Endergonic transformations or processes can proceed in the forward direction only by coupling to an exergonic process with a driving force more negative than the positive force of the endergonic process. The backward direction of an endergonic process is exergonic. The distinction between endergonic and endothermic processes is at the heart of ergodynamics, emphasising the concept of exergy changes, linked to the performance of work, in contrast to enthalpy changes, linked to heat or thermal processes, the latter expression being terminologically linked to thermodynamics. | |
| Endothermic | An energy transformation is endothermic if the enthalpy change of a closed system is positive when the process takes place in the forward direction and heat is absorbed from the environment under isothermal conditions (∆eQ > 0) without performance of work (∆eW = 0). The same energy transformation is exothermic if it proceeds in the backward direction. Exothermic and endothermic transformations can proceed spontaneously without coupling only, if they are exergonic. | |
| Endothermy | Endothermy is the constant regulation of body temperature by metabolic heat production and control of heat exchange with the environment. | |
| Energy | E; various [J] | Heat and work are forms of energy [1 cal = 4.184 J]. Energy [J] is a fundamental term that is used in physics and physical chemistry with various meanings [1]. These meanings become explicit in the following equations relating to systems at constant volume (dV = 0) or constant gas pressure (dp = 0). Energy is exchanged between a system and the environment across the system boundaries in the form of heat, deQ, total or available work, detW (or detW), and matter, dmatU (or dmatH) [2], dU = (deQ + detW) + dmatU ; dV = 0 [Eq. 1a] dH = (deQ + deW) + dmatH ; dp = 0 [Eq. 1b] Whereas dU (or dH) describe the internal-energy change (or enthalpy change) of the system, heat and work are external energy changes (subscript e; et: external total; e: external excluding pressure-volume work), and dmatU (or dmatH) are the exchange of matter expressed in internal-energy (or enthaply) equivalents. In closed systems, dmatU = 0 (dmatH = 0). The energy balance equation [Eq. 1] is a form of the First Law of Thermodynamics, which is the law of conservation of internal-energy, stating that energy cannot be generated or destroyed: energy can only be transformed into different forms of work and heat, and transferred in the form of matter. Notably, the term energy is general and vague, since energy may be associated with either the first or second law of thermodynamics. Work is a form of energy exchange [Eq. 1], but can be seen as exergy exchange in conjunction with deG = deW in a closed system [Eq. 3b]. An equally famous energy balance equation considers energy changes of the system only, in the most simple form for isothermal systems (dT = 0): dU = dA + T∙dS = dU + dB [Eq. 2a] dH = dG + T∙dS = dG + dB [Eq. 2b] The internal-energy change, dU (enthalpy change, dH) is the sum of free energy change (Helmholtz energy, dA; or Gibbs energy = exergy change, dG) and bound energy change (bound energy, dB = T∙dS). The bound energy is that part of the energy change that is always bound to an exchange of heat. A third energy balance equation accounts for changes of the system in terms of irreversible internal processes (i) occuring within the system boundaries, and reversible external processes (e) of transfer across the system boundaries (at constant gas pressure), dH = diH + deH [Eq. 3a] dG = diG + deG [Eq. 3b] The energy conservation law of thermodynamics (first law) can be formulated as diH = 0 (at constant gas pressure), whereas the generally negative sign of the dissipated energy, diG ≡ diD ≤ 0, is a formulation of the second law of thermodynamics. Insertion into Eq. 3 yields, dH = deH [Eq. 4a] dG = diD + deW + dmatG [Eq. 4b] When talking about energy transformations, the term energy is used in a general sense without specification of these various forms of energy. |
| Energy charge | AEC | The energy charge of the adenylate system or adenylate energy charge (AEC) has been defined by Atkinson and Walton (1967) as (ATP + ½ ADP)/(AMP + ADP + ATP). Wheather the AEC is a fundamental metabolic control parameter remains a controversial topic. |
| Energy metabolism | Core energy metabolism is the integrated biochemical process supplying the cell with ATP, utilizing ATP for various forms of work including biogenesis, maintaining ion and redox balance, and in specific organisms or tissues dissipating heat for temperature regulation. | |
| Energy saving in research | Energy saving in research must rank as a priority of social responsibility — ever since the Club of Rome published 50 years ago the seminal book on The limits to growth (1972) [1], and more so today in face of the global threat of climate change and the russian war in aggression against Ukraine. Energy saving in research does not and must not clash with quality in research. Application of high-quality and predefined experimental protocols combined with evaluation of repeatability and reproducibility represents primary strategies for energy saving in research. Publication of irreproducible results — adding to the reproducibility crisis — is the most wasteful aspect of research in terms of resources including energy (more properly: exergy). Paywall journalism is wasteful in terms of financial resources. Dramatically increasing numbers of scientific publications is a pathway towards waste of energy [2]. Besides large-scale strategies on e(n)xergy saving in research — quality versus quantity —, everybody's everyday contributions to energy saving count: to cut greenhouse gas emissions, save biological and geological diversity, and improve equality across societies, gender, continents, and countries. Do scientists take responsibility for energy saving? Or does biomedical research merely find excuses? Scientific institutions in academia and industry must implement energy saving strategies to reduce waste according to the European Union's Energy efficiency directive, and to consume less energy (exergy) by using it more efficiently (Energy efficiency targets). Possible — important but much neglected — contributions include:
| |
| Enthalpy | H [J] | Enthalpy, H [J], can under conditions of constant gas pressure neither be destroyed nor created (first law of thermodynamics: diH/dt = 0). The distinction between enthalpy and internal-energy of a system is due to external pressure-volume work carried out reversibly at constant gas pressure. The enthalpy change of the system, dH, at constant pressure, is the internal-energy change, dU, minus reversible pressure-volume work, dH = dU - dVW Pressure-volume work, dVW, at constant pressure, is the gas pressure, p [Pa = J·m-3], times change of volume, dV [m3], dVW = -p·dV [J] The available work, deW, is distinguished from external total work, detW, [1] deW = detW - dVW The change of enthalpy of a system is due to internal and external changes, dH = diH + deH Since diH = 0 (first law of thermodynamics), the dH is balanced by exchange of heat, work, and matter, dH = (deQ + deW) + dmatH ; dp = 0 The exchange of matter is expressed in enthalpy equivalents with respect to a reference state (formation, f, or combustion, c). The value of dH in an open system, therefore, depends on the arbitrary choice of the reference state. In contrast, the terms in parentheses are the sum of all (total, t) partial energy transformations, dtH = (deQ + deW) A partial enthalpy change of transformation, dtrH, is distinguished from the total enthalpy change of all transformations, dtH, and from the enthalpy change of the system, dH. In a closed system, dH = dtH. The enthalpy change of transformation is the sum of the Gibbs energy (free energy) change of transformation, dtrG, and the bound energy change of transformation at constant temperature and pressure, dtrB = T·dS, dtrH = dtrG + dtrB |
| Entity | X | An entity of type X is something that can measured as an extensive quantity or counted as an elementary entity. The term entity with symbol X, therefore, has a general meaning, including but not limited to elementary entities UX. The distinction can be emphasized by using the term entity-type X, to avoid confusion of an entity X with the more restricted definition of elementary entity UX as a single countable object or event. |
| Equality | = | Physicochemical equality (symbol =) indicates in an equation not only numerical equivalence (symbol ≡), but an identity of the full meaning. |
| Equivalence | ≡ | Numerical equivalence (symbol ≡) indicates that two quantities are numerically equal, even if the full meaning may be different. For instance: 1 ≡ 1·1 and 1 ≡ 1/1. In contrast to ≡, the symbol = indicates physicochemical equality. |
| Ergodynamic efficiency | ε | The ergodynamic efficiency, ε (compare thermodynamic efficiency), is a power ratio between the output power and the (negative) input power of an energetically coupled process. Since power [W] is the product of a flow and the conjugated thermodynamic force, the ergodynamic efficiency is the product of an output/input flow ratio and the corresponding force ratio. The efficiency is 0.0 in a fully uncoupled system (zero output flow) or at level flow (zero output force). The maximum efficiency of 1.0 can be reached only in a fully (mechanistically) coupled system at the limit of zero flow at ergodynamic equilibrium. The ergodynamic efficiency of coupling between ATP production (DT phosphorylation) and oxygen consumption is the flux ratio of DT phosphorylation flux and oxygen flux (P»/O2 ratio) multiplied by the corresponding force ratio. Compare with the OXPHOS-coupling efficiency. |
| Ergodynamics | The mission of ergodynamics is the revelation of relations of general validity. "Thermodynamics deals with relationships between properties of systems at equilibrium and with differences in properties between various equilibrium states. It has nothing to do with time. Even so, it is one of the most powerful tools of physical chemistry" [1]. Ergodynamics is the theory of exergy changes (from the Greek word 'erg' which means work). Ergodynamics includes the fundamental aspects of thermodynamics ('heat') and the thermodynamics of irreversible processes (TIP; nonequilibrium thermodynamics), and thus links thermodynamics to kinetics. In its most general scope, ergodynamics is the science of energy transformations. Classical thermodynamics includes open systems, yet as a main focus it describes closed systems. This is reflected in a nomenclature that is not easily applicable to the more general case of open systems [2]. At present, IUPAC recommendations [3] fall short of providing adequate guidelines for describing energy transformations in open systems. | |
| Ethanol | ethanol abs. |
Ethanol or ethyl alcohol, C2H6O or EtOH, is widely used in the laboratory, particularly as a solvent and cleaning agent. There are different grades of high purity ethanol. Up to a purity of 95.6 % ethanol can be separated from water by destillation. Higher concentrations than 95% require usage of additives that disrupt the azeotrope composition and allow further distillation. Ethanol is qualified as "absolute" if it contains no more than one percent water. Whenever 'ethanol abs.' is mentioned without further specification in published protocols, it refers to ≥ 99 % ethanol a.r. (analytical reagent grade).
|
| Ethics on publishing | Ethics on publishing follow COPE's guidelines (or equivalent). A journal's policy on publishing ethics should be clearly visible on its website, and should refer to: (1) Journal policies on authorship and contributorship; (2) How the journal will handle complaints and appeals; (3) Journal policies on conflicts of interest / competing interests; (4) Journal policies on data sharing and reproducibility; (5) Journal's policy on ethical oversight; (6) Journal's policy on intellectual property; and (7) Journal's options for post-publication discussions and corrections. | |
| Ethylene glycol tetraacetic acid | EGTA | Ethylene glycol tetraacetic acid (EGTA) is a chelator for heavy metals, with high affinity for Ca2+ but low affinity for Mg2+. Sigma E 4378. |
| Etomoxir | Eto | Etomoxir (Eto; 2[6(4-chlorophenoxy)hexyl]oxirane-2-carboxylate) is an irreversible inhibitor of carnitine palmitoyltransferase I (CPT-I) on the outer face of the mitochondrial inner membrane. Eto inhibits fatty acid oxidation by blocking the formation of acyl carnitines from long-chain fatty acids which require the carnitine shuttle for transport into mitochondria. In contrast to long-chain fatty acids, the transport of short- and medium-chain fatty acids is carnitine-independent. |
| European Bioenergetics Conference |  EBEC is a group based in Europe that organizes the European Bioenergetics Conference.
EBEC is a group based in Europe that organizes the European Bioenergetics Conference. | |
| Euthanyl/Pentobarbitol | Euthanyl | I am often asked by reviewers to discuss the effects of pentobarbitol euthansia on mithochondrial function. Takaki 1997 JJP: This paper has been helpful in this discussion. (edit by Staples JF) |
| Events - DatLab | F4 | An event in DatLab is a defined point in time, labelled by a name (1 to 10 characters). An event applies to all plots of the selected O2k-Chamber. The event is shown by a vertical line in the graph and the label of the event is shown on the top (DatLab 6 and lower: on the bottom). The default name is the sequential number of the event. It is recommended to edit event labels with a minimum number of characters, and to explain the abbreviation in the 'Definition' box. The final concentration and titration volume can be entered into the corresponding boxes, if the event relates to the titration of a substance. A short comment can be entered to describe the event in detail. Set events - Manual events are entered (real-time, connected to the O2k) by pressing [F4] at the time of the event (e.g. to indicate a manual titration into the chamber). An event belongs either to chamber A, chamber B, or both. Instrumental events are added automatically, e.g. when the stirrer (A or B) or illumination (both chambers) is switched on or off. After setting a new event the Edit event window pops up. Pressing F4 defines the time point of the event. Full attention can then be paid to the experiment. Edit the event later, as it is possible to insert an event at any chosen moment of the plotted record of the experiment by placing the cursor anywhere in the graph at the selected time point by pressing Ctrl and clicking the left mouse button. Edit event - Left click on the name of an existing event to open the Edit event window to edit or Delete event. In events obtained from a selected protocol, the entire sequence of consecutive events is defined with event names, definitions, concentrations and titration volumes. Name - Enter an event name of 1 to 10 characters. Short names (e.g. O instead of Open) are recommended. Comment - Further information can be entered into the text field. Select O2k-chamber A, B or both. The Event will be shown on plots for both or one selected chamber. »Protocol events |
| Examination | An examination is a set of operations having the object of determining the value or characteristics of a property. In some disciplines (e.g. microbiology) an examination is the total activity of a number of tests, observations or measurements. | |
| Exclusion criteria | The Exclusion criteria include factors or characteristics that make the recruited population ineligible for the outcome parameter. With the Inclusion criteria, this factor must be a cofounder for the outcome parameter | |
| Exergonic | Exergonic transformations or processes can spontaneously proceed in the forward direction, entailing the irreversible loss of the potential to performe work (erg) with the implication of a positive internal entropy production. Ergodynamic equilibrium is obtained when an exergonic (partial) process is compensated by a coupled endergonic (partial) process, such that the Gibbs energy change of the total transformation is zero. Final thermodynamic equilibrium is reached when all exergonic processes are exhausted and all forces are zero. The backward direction of an exergonic process is endergonic. The distinction between exergonic and exothermic processes is at the heart of ergodynamics, emphasising the concept of exergy changes, linked to the performance of work, in contrast to enthalpy changes, linked to heat or thermal processes, the latter expression being terminologically linked to thermodynamics. | |
| Exergy | E; various [J] | Exergy includes external and internal work. Exergy as the external work is defined in the First Law of thermodynamics as a specific form of energy. Exergy as the dissipated Gibbs or Helmholtz energy is the irreversibly dissipated (internal) loss of the potential of performing work as defined in the Second Law of Thermodynamics. Changes of exergy dG plus bound energy yield the enthalpy change: dH = dG + T∙dS = dG + dB |
| Exit - DatLab 7 | Ctrl+F4 | Close DatLab files and quit the program. |
| Exothermic | An energy transformation is exothermic if the enthalpy change of a closed system is negative when the process takes place in the forward direction and heat is lost to the environment under isothermal conditions (∆eQ < 0) without performance of work (∆eW = 0). The same energy transformation is endothermic if it proceeds in the backward direction. Exothermic and endothermic transformations can proceed spontaneously without coupling only, if they are exergonic. | |
| Experiment | A number of replica, N, of experiments on one sample type is designed to obtain statistical information about the involved population(s) and to test hypotheses about a population and about differences between populations, when experiments are carried out on different sample types. An experiment may involve various assays, e.g., a respirometric assay and an assay for protein determination. | |
| Experimental code | F3 | An experimental code can be entered in the Sample window, containing up to 10 digits. |
| Experimental log - DatLab | Ctrl+F3 | Experimental log provides an automatically generated experimental protocol with detailed information about the O2k settings and calibrations, the Sample information and various Events. Time-dependent information can be viewed for a single chamber or both chambers. The filter can be selected for viewing minimum information, intermittent by default, or all information. The experimental log can be viewed and saved as a PDF file by clicking on [Preview]. |
| Export DL-Protocol User (*.DLPU) | it is a function of DatLab (available from version 7.4 onwards) that enables the export of user specific protocols (DL-Protocol User) to the SUIT protocol folder from which they can be uploaded for subsequent measurements. | |
| Export as CSV - DatLab | Ctrl+E | Export as CSV (*.csv) exports plots and events to a text file for further use in Excel and other programs compatible with .csv extension. |
| Extended abstracts | In the context of MiPevents, extended abstracts are accepted for preprint publication in MitoFit Preprints upon evaluation by the MitoFit Preprints Scientific Advisory Board. Publishing extended abstracts with MitoFit Preprints does not preclude later full journal publication, but will make your work fully citable, by assigning each manuscript a unique DOI number, and facilitate discovery and feedback. | |
| Extensive quantity | Extensive quantities pertain to a total system, e.g. oxygen flow. An extensive quantity increases proportional with system size. The magnitude of an extensive quantity is completely additive for non-interacting subsystems, such as mass or flow expressed per defined system. The magnitude of these quantities depends on the extent or size of the system (Cohen et al 2008). | |
| External flow | Ie [MU·s-1] | External flows across the system boundaries are formally reversible. Their irreversible facet is accounted for internally as transformations in a heterogenous system (internal flows, Ii). |