Semantic search
| Term | Abbreviation | Description |
|---|---|---|
| O2k-Chamber Holder | O2k-Chamber Holder (blue POM) for PVDF or PEEK stoppers (2-mL O2k-chamber), with O-ring\Viton\18x2 mm and V-ring\30-35-4.5 mm. Two units of this item are standard components mounted on the O2k-Main Unit. | |
| O2k-Chamber Holder sV | O2k-Chamber Holder sV (black POM) for PVDF or PEEK stoppers (0.5-mL O2k-chamber), with O-ring\Viton\16x2 mm and V-ring\30-35-4.5 mm. | |
| O2k-Chamber sV | O2k-Chamber sV: 12 mm inner diameter, Duran® glass polished, with standard operation volume V of 0.5 mL. | |
| O2k-Dissection Set | O2k-Dissection Set: for tissue preparation, set of 4 pairs of stainless steel, antimagnetic forceps and a pair of scissors. | |
| O2k-Electromagnetic Stirrer Twin-Control | O2k-Electromagnetic Stirrer Twin-Control for smooth rotation of the stirrer bars in the two O2k-chambers; with slow-start function to prevent decoupling of the stirrer bar; regulated stirrer speed in the range of 100 to 800 rpm (decoupling may occur at higher stirrer speeds), independent for the two O2k-Chambers; automatic events sent to DatLab when the stirrer is switched on/off or when the rotation seed is changed by the experimenter. Integral component of the O2k-Main Unit. | |
| O2k-Fluo LED2-Module | The O2k-Fluo LED2-Module is a component of the O2k-Fluorometer (O2k-Series D to G). It is an amperometric add-on module to the O2k-Core (O2k-Series D to G), adding a new dimension to high-resolution respirometry. Optical sensors are inserted through the front window of the O2k-glass chambers, for measurement of hydrogen peroxide production (Amplex® UltraRed), ATP production (Magnesium Green™), mt-membrane potential (Safranin, TMRM, Rhodamine 123), Ca2+ (Calcium Green™), and numerous other applications open for O2k-user innovation. | |
| O2k-Fluo Smart-Module | The O2k-Fluo Smart-Module is an amperometric add-on module to the O2k-Respirometer, adding a new dimension to high-resolution respirometry. Optical sensors are inserted through the front window of the O2k-glass chambers, for measurement of hydrogen peroxide production (Amplex® UltraRed), ATP production (Magnesium Green™), mt-membrane potential (Safranin, TMRM), Ca2+ (Calcium Green™), and numerous other applications open for O2k-user innovation. | |
| O2k-FluoRespirometer | The Oroboros O2k-FluoRespirometer - the experimental system complete for high-resolution respirometry (HRR), including fluorometry, the TIP2k and the O2k-sV-Module allowing simultaneous monitoring of oxygen consumption together with either ROS production (AmR), mt-membrane potential (TMRM, Safranin and Rhodamine 123), Ca2+ (CaG) or ATP production (MgG). The O2k-FluoRespirometer supports all add-on O2k-Modules: O2k-TPP+ ISE-Module, O2k-pH ISE-Module, O2k-NO Amp-Module, enabling measurement of mt-membrane potential with ion sensitive electrodes (ISE for TPP+ or TPMP+) or pH. | |
| O2k-Fluorometer Series G | O2k-Fluorometer Series G - Former Series (up to 2017-July) - the experimental system complete for high-resolution respirometry (HRR) combined with fluorometry. The O2k-Fluorometer includes the O2k-Core, O2k-Fluo LED2-Module and TIP2k, and supports all other add-on O2k-Modules of the Oroboros O2k. The O2k is a sole source apparatus with no other instruments meeting its test experiments on O2k-Specifications. | |
| O2k-Fuse Power Plug\M2.5 A\5x20 mm | O2k-Fuse Power Plug\M2.5 A\5x20 mm: This item is a standard component of the O2k-Assembly Kit (O2k-FluoRespirometer), mounted on the socket for the O2k-Main Power Cable, at the rear panel of the O2k-Main Unit. | |
| O2k-Main Basic | The O2k-Main Basic is an integral element of the O2k-Main Unit. The Oroboros O2k Main Basic has the following components:
| |
| O2k-Main Power Cable | O2k-Main Power Cable, for connecting the main unit to the power supply. | |
| O2k-Main Power Cable\120 V\US-CA | O2k-Main Power Cable\120 V\US-CA, USA and Canada (120 V). | |
| O2k-Main Power Cable\230 V\AU-NZ | O2k-Main Power Cable\AU-NZ, Australia and New Zealand (230 V). | |
| O2k-Main Power Cable\230 V\Europe | O2k-Main Power Cable\230 V\Europe. | |
| O2k-Main Unit | The O2k-Main Unit is a component of the O2k-Core. The O2k-Main Unit consists of functionally defined, integral elements, the (O2k-Main Basic, O2k-Peltier Temperature Control, two O2k-Electromagnetic Stirrer Twin-Control units, two O2k-Amperometric OroboPOS Twin-Channels, O2k-Barometric Pressure Transducer), which cannot be obtained separately. | |
| O2k-MultiSensor | When one (or more) analytical parameters are monitored simultaneously with oxygen concentration and oxygen flux, this is an O2k-MultiSensor application of the Oroboros O2k-technology. The NextGen-O2k supports all O2k-MultiSensor Modules, while the O2k does not provide for the Q- and NADH-Redox-Modules. For some O2k-MultiSensor applications it is necessary to introduce one or more additional sensors into the chamber through a MultiSensor stopper. Optical applications require the standard black stoppers. | |
| O2k-NO Amp-Module | O2k-NO Amp-Module: NO-sensor compatability pack an amperometric add-on for O2k-MultiSensor application The NO sensor is not included. | |
| O2k-Network Reference Laboratory | O2k-Network Lab |
O2k-Network Reference Laboratories build a WorldWide network on high-resolution respirometry and mitochondrial physiology, the Oroboros O2k-Network. |
| O2k-Open Support agreement | O2k-Open Support aims at providing expert help quickly. Please, help us sharing our support communication openly with the scientific community. | |
| O2k-Peltier Temperature Control | O2k-Peltier Temperature Control: Built-in electronic thermostat controlling temperature for two O2k-chambers in the range of 4 to 47 °C; ±0.002 °C (at room temperature). Continuous recording of the O2k-Copper Block temperature with DatLab. Temperature change from 20 to 30 °C within 15 min; cooling from 30 to 20 °C within 20 min. Integral component of the O2k-Main Unit. The electronic temperature control of the O2k replaced the conventional water jacket. | |
| O2k-Publications: Exercise physiology;nutrition;life style | ||
| O2k-Publications: Obesity | ||
| O2k-Specifications | O2k versus multiwell respirometer: O2k stands for Oroboros O2k and high-resolution respirometry, meeting powerful quality criteria securing high output and pioneering state-of-the-art comprehensive OXPHOS analysis of substrate control and coupling control of mitochondrial function. 'High throughput' stands for disposable multiwell systems - expensive, with limited scope and extremely high running costs. In respirometry, high throughput is not equivalent to high output. If you’re using a biased instrument, it doesn’t matter how many measurements you take – you’re aiming at the wrong target (Silver 2012 Penguin Press). | |
| O2k-TPP+ ISE-Module | O2k-TPP+ ISE-Module: Potentiometric ion-selective electrodes for measurement of mitochondrial membrane potential | |
| O2k-Titration Set | The O2k-Titration Set consists of Hamilton microsyringes (6 x 10 mm3 and 3 spare plungers, 6 x 25 mm3, 1 x 50 mm3, 1 x 100 mm3, 1 x 500 mm3; fixed needles with rounded tips), provided in the Syringe Storage Box with Syringe Labels, a set of two Syringe Racks with Syringe Collars, and a set of two Tube Racks. | |
| O2k-USB Flash Drive | The O2k-USB Flash Drive is a component of the Oroboros O2k containing: DatLab, O2k-Manual, O2k-Protocols, O2k-Publications, and info on O2k-Workshops. | |
| O2k-Virtual Support | O2k-Virtual support includes 8 individual hours. Via a live video link, Oroboros experts guide you step-by-step on topics of your choice, such as O2k instrumental setup and service of the polarographic oxygen sensors (POS) for instrumental quality control, an essential component of HRR. This offers the opportunity to analyze and discuss your experimental DatLab files obtained with your O2k with the bioenergetics experts of Oroboros. It offers flexibility to participants and gives the option to choose virtual sessions that best fit individual needs. | |
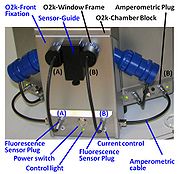
| O2k-Window Frame |
O2k-Window Frame: blue POM, with thread for fixation on the O2k-Main Unit, to be removed only for rare cleaning purposes and for front fixation of the Fluorescence-Control Unit, using the O2k-Window Tool. | |
| O2k-Window Tool |
O2k-Window Tool for removing the blue O2k-Window Frame from the O2k-Main Unit, for rare cleaning purposes and for front fixation of the Fluorescence-Control Unit. | |
| O2k-chamber |
O2k-Chamber: Duran® glass polished, with standard operation volumes (V) of 2.0 mL or 0.5 mL (small chamber volume in the O2k-sV-Module, 12 mm inner diameter). The optical properties of Duran® allow application of fluorometric sensors (Duran® optical properties). | |
| O2k-pH ISE-Module | O2k-pH ISE-Module: two pH electrodes and reference electrodes and accessories | |
| O2k-sV-Module | O2k-sV-Module |
The O2k-sV-Module is the O2k small-volume module, comprised of two Duran® glass chambers of 12 mm inner diameter specifically developed to perform high-resolution respirometry with reduced amounts of biological sample, and all the components necessary for a smaller operation volume V of 0.5 mL. The current DatLab version is included in the delivery of this revolutionary module. |
| O2k-ticket system | The O2k-ticket system is a customer support platform based on Zammad. This system automatically attributes an unique Ticket number (which is visible on the subject of your e-mail) to each received customer inquiry. For an easy follow-up, all the related correspondence is collected under this Ticket number.
In order to provide a helpful and reliable support regarding your O2k/equipment, we suggest to include in your inquiries:
| |
| OSF Preprint server | Leading preprint service providers use OSF Preprints as an open source infrastructure to support their communities. You should upload your preprint to whichever preprint server best fits your topic and the community that you would like to reach. If there isn’t a community-driven preprint server for your discipline, OSF Preprints is available for any discipline. Currently, you can only share your preprint on one community preprint server. It’s on our roadmap to allow users to submit a preprint to multiple community preprint servers. However, to improve discoverability across communities, all preprints shared on OSF Preprints and community preprint servers are indexed and searchable via osf.io/preprints. Right now, it is not possible to add subjects. However, you can add tags with additional subject areas or keywords to improve discoverability. COS supports communities operating their own branded community preprint services using OSF Preprints as the backend.OSF is based in Charlottesville, VA, USA. | |
| OXPHOS International | The OXPHOS International web portal is a repository of information useful to scholars studying mitochondria. The site is operated as a private "special interests" community hub. | |
| OXPHOS capacity | P | |
| Obesity | Obesity is a disease resulting from excessive accumulation of body fat. In common obesity (non-syndromic obesity) excessive body fat is due to an obesogenic lifestyle with lack of physical exercise ('couch') and caloric surplus of food consumption ('potato'), causing several comorbidities which are characterized as preventable non-communicable diseases. Persistent body fat excess associated with deficits of physical activity induces a weight-lifting effect on increasing muscle mass with decreasing mitochondrial capacity. Body fat excess, therefore, correlates with body mass excess up to a critical stage of obesogenic lifestyle-induced sarcopenia, when loss of muscle mass results in further deterioration of physical performance particularly at older age. | |
| OctGM | OctGM | OctGM: Octanoylcarnitine & Glutamate & Malate. MitoPathway control state: FN |
| OctGMS | OctGMS | OctGMS: Octanoylcarnitine &Glutamate & Malate& Succinate. MitoPathway control state: FNS |
| OctM pathway control state | OctM | OctM: Octanoylcarnitine & Malate. MitoPathway control state: F SUIT protocols: SUIT-002, SUIT-015, SUIT-016, SUIT-017 Respiratory stimulation of the FAO-pathway, F, by fatty acid FA in the presence of malate M. Malate is a type N substrate (N), required for the F-pathway. In the presence of anaplerotic pathways (e.g., mitochondrial malic enzyme, mtME) the F-pathway capacity is overestimated, if there is an added contribution of NADH-linked respiration, F(N) (see SUIT-002). The FA concentration has to be optimized to saturate the FAO-pathway, without inhibiting or uncoupling respiration. Low concentration of malate, typically 0.1 mM, does not saturate the N-pathway; but saturates the F-pathway. High concentration of malate, typically 2 mM, saturates the N-pathway. |
| OctPGM pathway control state | OctPGM | OctPGM: Octanoylcarnitine & Pyruvate & Glutamate & Malate. MitoPathway control state: FN SUIT protocols: SUIT-002
|
| OctPGMS pathway control state | OctPGMS | OctPGMS: Octanoylcarnitine & Pyruvate & Glutamate & Malate & Succinate. MitoPathway control state: FNS SUIT protocol: SUIT-001, SUIT-002, SUIT-015 This substrate combination supports convergent electron flow to the Q-junction. |
| OctPGMSGp pathway control state | OctPGMSGp | OctPGMSGp: Octanoylcarnitine & Pyruvate & Glutamate & Malate & Succinate & Glycerophosphate. MitoPathway control state: FNSGp SUIT protocol: SUIT-002 This substrate combination supports convergent electron flow to the Q-junction. |
| OctPM pathway control state | OctPM | OctPM: Octanoylcarnitine & Pyruvate & Malate. MitoPathway control state: FN SUIT protocol: SUIT-002, SUIT-005 This substrate combination supports N-linked flux which is typically higher than FAO capacity (F/FN<0 in the OXPHOS state). In SUIT-RP1, PMOct is induced after PM(E), to evaluate any additive effect of adding Oct. In SUIT-RP2, FAO OXPHOS capacity is measured first, testing for the effect of increasing malate concentration (compare malate-anaplerotic pathway control state, M alone), and pyruvate is added to compare FAO as the background state with FN as the reference state. |
| OctPMS | OctPMS | OctPMS: Octanoylcarnitine & Pyruvate & Malate & Succinate. MitoPathway control state: FNS SUIT protocol: SUIT-005 |
| Octanoate | Oca | Octanoate (octanoic acid). C8H16O2 Common name: Caprylic acid. |
| Octanoylcarnitine | Oct | Octanoylcarnitine is a medium-chain fatty acid (octanoic acid: eight-carbon saturated fatty acid) covalently linked to carnitine, frequently applied as a substrate for fatty acid oxidation (FAO) in mitochondrial preparations. |
| Oligomycin | Omy | Oligomycin (Omy) is an inhibitor of ATP synthase by blocking its proton channel (Fo subunit), which is necessary for oxidative phosphorylation of ADP to ATP (energy production). The inhibition of ATP synthesis also inhibits respiration. In OXPHOS analysis, Omy is used to induce a LEAK respiration state of respiration (abbreviated as L(Omy) to differentiate from L(n), LEAK state in the absence of ADP). |
| Open - DatLab | Ctrl+O | Open a previously recorded DatLab file. |
| Open Access | OA | Open Access (OA) academic articles comprise all different forms of published research that are distributed online, free of charge and with an open license to facilitate the distribution and reuse. The open access repositories serve as the perfect vehicle to transmit free knowledge, including but not limited to peer-reviewed and non-peer-reviewed academic journal articles, conference papers, theses, book chapters and monographs. Driven by the problems of social inequality caused by restricting access to academic research, the Open Access movement changes the funding system of published literature allowing for more readers and thus increased access to scientific knowledge, as well as addressing the economic challenges and unsustainability of academic publishing. In addition to being free to read (gratis), open access articles may also be free to use (libre) where the copyright is held by the authors and not the publisher. Definition by the Directory of Open Access Journals (DOAJ): "We define these as journals where the copyright holder of a scholarly work grants usage rights to others using an open license (Creative Commons or equivalent) allowing for immediate free access to the work and permitting any user to read, download, copy, distribute, print, search, or link to the full texts of articles, crawl them for indexing, pass them as data to software, or use them for any other lawful purpose." |
| Open Science | OS | Building on the essential principles of academic freedom, research integrity and scientific excellence, open science sets a new paradigm that integrates into the scientific enterprise practices for reproducibility, transparency, sharing and collaboration resulting from the increased opening of scientific contents, tools and processes. Open science is defined as an inclusive construct that combines various movements and practices aiming to make multilingual scientific knowledge openly available, accessible and reusable for everyone, to increase scientific collaborations and sharing of information for the benefits of science and society, and to open the processes of scientific knowledge creation, evaluation and communication to societal actors beyond the traditional scientific community. It comprises all scientific disciplines and aspects of scholarly practices, including basic and applied sciences, natural and social sciences and the humanities, and it builds on the following key pillars: open scientific knowledge, open science infrastructures, science communication, open engagement of societal actors and open dialogue with other knowledge systems. |
| Open chamber | O | The term "open O2k-chamber" refers to a situation in which the liquid phase is allowed to equilibrate with a gas phase, but the stopper is partially inserted using the Stopper-Spacer. |
| Open system | An open system is a system with boundaries that allow external exchange of energy and matter; the surroundings are merely considered as a source or sink for quantities transferred across the system boundaries (external flows, Iext). | |
| Optics | Optics are the components that are used to relay and focus light through a spectrofluorometer or spectrophotometer. These would normally consist of lenses and/or concave mirrors. The number of such components should be kept to a minimum due to the losses of light (5-10%) that occur at each surface. | |
| Ordinate | y | The ordinate is the vertical axis y of a rectangular two-dimensional graph with the abscissa x as the horizontal axis. Values Y are placed vertically from the origin. |
| OroboPOS |
The OroboPOS is a polarographic oxygen sensor (POS), with an amperometric mode of operation. The OroboPOS meets the highest quality criteria in terms of linearity, stability and sensitivity of the signal. The Clark type polarographic oxygen sensor (POS) remains the gold standard for measuring dissolved oxygen in biomedical, environmental and industrial applications over a wide dynamic oxygen range. It consists of a gold cathode, a silver/silverchloride anode and a KCl electrolyte reservoir separated from the sample by a 25 µm membrane (FEP). The main body of the OroboPOS is made of PEEK. With application of a polarization voltage (0.8 V), a current is obtained as an amperometric signal, which is converted to a voltage. | |
| OroboPOS-Connector | OroboPOS-Connector (blue POM), with male connection to OroboPOS head (POS) and with cable and male plug fitting into O2k-Main Unit. | |
| OroboPOS-Connector Service | The OroboPOS-Connector Service entails routine maintenance and any necessary repairs of the OroboPOS-Connector in the Oroboros electronics workshop (WGT). | |
| Oroboros Instruments Corp |
| |
| Oroboros O2k-Core (O2k-Series D - G) | Oroboros O2k-Core - Former Series (O2k-Series D - G) - the experimental system complete for basic high-resolution respirometry (HRR). The O2k-Core includes the O2k-Main Unit with stainless steel housing, O2k-Assembly Kit, two OroboPOS (polarographic oxygen sensors) and OroboPOS-Service Kit, DatLab software, the ISS-Integrated Suction System and the O2k-Titration Set. The O2k-Core supports all add-on O2k-Modules of the O2k. On-line display of oxygen flux (rate of respiration) is provided in addition to the conventional 'oxygraphic' plot of oxygen concentration over time. Highest signal stability minimizes the required amounts of biological sample, and provides the basis for resolution in the extreme low-oxygen range. Peltier temperature control provides a thermal stability at ±0.002 °C in the range of 4 °C to 47 °C at typical constant room temperature. Electronically controlled PVDF or PEEK stirrers are integrated in the two-chamber design of the O2k, and a barometric pressure transducer enables automatic oxygen calibrations implemented in the DatLab software. The O2k is a sole source apparatus with no other instruments meeting its specifications. | |
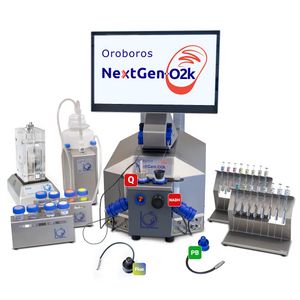
| Oroboros O2k-technology |
The Oroboros O2k-technology provides modular systems for high-resolution respirometry (HRR) for mitochondria and cell research. Oroboros delivers the O2k-technology for high-resolution respirometry in mitochondria and cell research. The O2k-tecnology allows the measurement of respiration at controlled oxygen levels, combined with redox biology (NADH and CoQ), ROS production, mitochondrial membrane potential, ATP production, Ca2+, or pH. HRR expands to HRPB: High-Resolution PhotoBiology. Small amounts of biological samples can be used for bioenergetic and OXPHOS analysis, ranging from isolated mitochondria, permeabilized tissues and permeabilized cells to living cells and tissues slices. The modular O2k-concept is supported by DatLab, with high flexibility for extension by add-on O2k-Modules. All O2k-Modules are supported by the NextGen-O2k. The O2k-Q-Module and the O2k-NADH-Module are exclusively supported by the NextGen-O2k, whereas the O2k (Series-J) provides the basis for all other HRR application but cannot be upgraded to the NextGen-O2k Redox. The globally tested and trusted high-resolution O2k-technology prioritizes both quality and scientific research output in the field of mitochondrial physiology and pathology, extended to PhotoBiology. | |
| Oroboros USB-flash drive | The Oroboros USB-flash drive is delivered with the Oroboros O2k. Copy the folder "Oroboros O2k-Course on HRR" from the Oroboros USB-flash drive to your computer. This folder contains the DatLab installation program as well as tools to find topics, O2k-manuals and O2k-protocols with corresponding DatLab demo files and templates for training with DatLab. | |
| Ouabain | Oua | Ouabain (synonym: G-strophantin octahydrate) is a poisonous cardiac glycoside. The classical mechanism of action of ouabain involves its binding to and inhibition of the plasma membrane Na+/K+-ATPase (sodium pump) especially at the higher concentrations. Low (nanomolar and subnanomolar) concentrations of ouabain stimulate the Na-K-ATPase. |
| Outlier | An outlier is a member of a set of values which is inconsistent with other members of that set. An outlier can arise by chance from the expected population, originate from a different population, or be the result of an incorrect recording or other blunder. Many schemes use the term outlier to designate a result that generates an action signal. This is not the intended use of the term. While outliers will usually generate action signals, it is possible to have action signals from results that are not outliers [SOURCE: ISO 5725‑1:1994, modified]. | |
| Outlier index threshold - DatLab | ||
| Outlier-skewness index | OSI, OI | An outlier-skewness index OSI is defined for evaluation of the distribution of data sets with outliers including separate clusters or skewness in relation to a normal distribution with equivalence of the average and median. The OSI is derived from Pearson’s coefficient of skewness 2:
The outlier-skewness index OSI introduces the absolute value of the arithmetic mean, m = ABS(average + median)/2, for normalization:
At the limit of a zero value of m, the OSI equals the Pearson 2 coefficient (without the multiplication factor of 3). At high m with small standard deviation (SD), the OSI is effectively the difference between the average and the median normalized for m, (average-median)/m. |
| Overfitting | Overfitting in statistics is the act of mistaking noise for a signal. Overfitting makes a model look ‘’better’’ on paper but perform ‘’worse’’ in the real world. This may make it easier to get the model published in an academic journal or to sell to a client, crowding out more honest models from the marketplace. But if the model is fitting noise, it has the potential to hurt the science (quoted from Silver 2012 Penguin Press). | |
| Overlay of plots - DatLab | Overlay of plots is defined in DatLab as selection of graph layouts showing identical plots from the two O2k-chambers in each graph. Overlay of plots is selected in Graph layout. Superimposed traces of flux/flow from chambers A and B are then shown in Graph 1, and of concentration in chambers A and B in Graph 2. There are basically two ways to superimpose traces recorded in different experiments: Export of the graphics via windows metafile or export of the data to e.g. a spreadsheet program. If you export via wmf you also can manipulate the graphics but then usually the lines are broken up in different segments. This can be done in various programs like MS Word, Open Office Draw and even in MSPower Point, though this maybe is the worst program to do this. It is better to manipulate them in a proper program like OO Draw, convert it to an unchangeable picture and then import it to a presentation graphics. Anyway, when you import directly to Power point (or other programs), make sure not to import it as a "picture" but as a metafile. Also in some programs you might afterwards have to "break" it up, or accept a "conversion to a MS Draw object" or other similar linguistic inventions of the software gurus. For this option we suggest to do as much as possible directly in DatLab (setting colors, line widths, ..) using the options in "Plots"/"select plots" and "graph"/"options". The “hardcore“ option is to export the data and import it into e.g. a spreadsheet program (MS Excel , OOCalc). It takes longer to have a simple overlay but gives you far less problems later and its easier to make changes later. To do this you can export your dataset "Export"/"Data to Textfile" and then go from there. | |
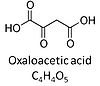
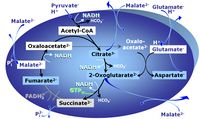
| Oxaloacetate | Oa |
Oxaloacetic acid, C4H4O5, occurs under physiological conditions as the anion oxaloacetate2-, Oa. Oxaloacetate is formed from malate by MDH. Oa reacts with acetyl-CoA through citrate synthase to form citrate, or with glutamate through transaminase to form oxoglutarate and aspartate. Oa transport is restricted across the inner mt-membrane of various tissues. Oa is a potent inhibitor of succinate dehydrogenase. |
| Oxalomalic acid | Oxalomalic acid is an inhibitor of aconitase (and of cytoplasmic NADP-dependent isocitrate dehydrogenase). Aconitase mediates the isomerization of citrate to isocitrate as the first step in the TCA cycle. Oxalomalic acid has been used at 1 mM concentration and after 45 min of pre-incubation to inhibit aconitase in permeabilized rat Soleus muscle fibres, inhibiting the enzyme by 24% (Osiki 2016 FASEB J). | |
| Oxia | Oxia - HyperOxia to HypOxia: The Oxia generates gaseous oxygen and hydrogen by electrolysis of water using a proton exchange membrane (PEM). O2 and H2 gas can be used to control the O2 regime in the Oroboros O2k (Setting_the_oxygen_concentration) using the 10 mL Gas-Injection Syringes. Low oxygen concentrations (<50 µM) are used to mimic tissue normoxia or hypoxia. Hyperoxic conditions above air saturation (250-600 µM O2) are routinely used for high-resolution respirometry of permeabilized muscle fibers or to induce oxidative stress in cells and mitochondrial preparations. | |
| Oxidative phosphorylation | OXPHOS | |
| Oxidative stress | Oxidative stress results from an imbalance between pro-oxidants and antioxidants shifting the equilibrium in favor of the pro-oxidants. This process can be due by an increment in pro-oxidants, by a depletion of antioxidant systems or both. Oxidative stress generates oxidative damage of proteins, lipids and DNA. | |
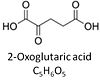
| Oxoglutarate | Og |
2-Oxoglutaric acid or alpha-ketoglutaric acid, C5H6O5, occurs under physiological conditions as the anion 2-Oxoglutarate2-, Og. 2-Oxoglutarate (alpha-ketoglutarate) is formed from isocitrate as a product of isocitrate dehydrogenase (IDH) in the TCA cycle, and is a substrate of oxoglutarate dehydrogenase (OgDH). The 2-oxoglutarate carrier exchanges malate2- for 2-oxoglutarate2- as part of the malate-aspartate shuttle. In the cytosol, oxoglutarate+aspartate are transaminated to form oxaloacetate+glutamate. Cytosolic malate dehydrogenase converts oxaloacetate+NADH to malate. |
| Oxoglutarate dehydrogenase | OgDH | Oxoglutarate dehydrogenase (α-ketoglutarate dehydrogenase) is a highly regulated enzyme of the tricarboxylic acid cycle. It catalyses the conversion of oxoglutarate (alpha-ketoglutarate) to succinyl-CoA, reduces NAD+ to NADH and thus links to Complex I in the Electron transfer-pathway. OgDH is activated by low Ca2+ (<20 µM) but inactivated by high Ca2+ (>100 µM). OgDH is an important source of ROS. |
| Oxycaloric equivalent | DeltakHO2 | The oxycaloric equivalent is the theoretically derived enthalpy change of the oxidative catabolic reactions per amount of oxygen respired, DeltakHO2, ranging from -430 to -480 kJ/mol O2. The oxycaloric equivalent is used in indirect calorimetry to calculate the theoretically expected metabolic heat flux from the respirometrically measured metabolic oxygen flux. Calorimetric/respirometric ratios (CR ratios; heat/oxygen flux ratios) are experimentally determined by calorespirometry. A CR ratio more exothermic than the oxycaloric equivalent of -480 kJ/mol indicates the simultaneous involvement of aerobic and anaerobic mechanisms of energy metabolism. |
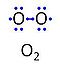
| Oxygen | O2 |
Molecular oxygen, O2 or dioxygen, has two atoms of oxygen, O, which is the chemical element with atomic number 8. The relative molecular mass of O2, Mr,O2, is 32 (or 31.9988). The element O has 8 protons, 8 neutrons and 8 electrons. In the figure, the two electrons in the first electron shell are not shown. Of the six electrons in the outer shell (blue bullets), one electron from each of the two atoms is shared in O2 forming the covalent bond, and one electron in each atom is unpaired. |
| Oxygen calibration - DatLab | O2 calibration is the calibration in DatLab of the oxygen sensor. It is a prerequisite for obtaining accurate measurements of respiration. Accurate calibration of the oxygen sensor depends on (1) equilibration of the incubation medium with air oxygen partial pressure at the temperature defined by the experimenter; (2) zero oxygen calibration; (3) high stability of the POS signal tested for sufficiently long periods of time; (4) linearity of signal output with oxygen pressure in the range between oxygen saturation and zero oxygen pressure; and (5) accurate oxygen solubility for aqueous solutions for the conversion of partial oxygen pressure into oxygen concentration. The standard oxygen calibration procedure is described below for high-resolution respirometry with the calibration routine using instrumental calibration DL-Protocols in DatLab. | |
| Oxygen flow | IO2 [mol·s-1] or [mol·s-1·x-1] | Respiratory oxygen flow is the oxygen consumption per total system, which is an extensive quantity. Flow is advancement of a transformation in a system per time [mol·s-1], when 'system' is defined as the experimental system (e.g. an open or closed chamber). Flow is distinguished from the size-specific quantity flux obtained by normalization of flow per volume of the experimental system [mol·s-1·m-3]. An experimental object, e.g. a living cell, may be considered as the 'experimental system'. Then oxygen flow per cell has the unit [mol·s-1·x-1], where [x] is the elementary unit for a count. Oxygen flow or respiration per cell [amol·s-1·x-1] = [pmol·s-1·Mx-1] is normalized for the cell count, distinguished from oxygen flux (e.g. per mg protein or wet mass). These are different forms of normalization of rate. |
| Oxygen flux | JO2 | Oxygen flux, JO2, is a specific quantity. Oxygen flux is oxygen flow, IO2 [mol·s-1 per system] (an extensive quantity), divided by system size. Flux may be volume-specific (flow per volume [pmol·s-1·mL-1]), mass-specific (flow per mass [pmol·s-1·mg-1]), or marker-specific (flow per mtEU). Oxygen flux (e.g., per body mass, or per cell volume) is distinguished from oxygen flow (per number of objects, such as cells), IO2 [mol·s-1·x-1]. These are different forms of normalization of rate. |
| Oxygen flux - instrumental background | J°O2 | Instrumental background oxygen flux, J°O2, in a respirometer is due to oxygen consumption by the POS, and oxygen diffusion into or out of the aqueous medium in the O2k-chamber. It is a property of the instrumental system, measured in the range of experimental oxygen levels by a standardized instrumental O2 background test. The oxygen regime from air saturation towards zero oxygen is applied generally in experiments with isolated mitochondria, and living or permeabilized cells. To overcome oxygen diffusion limitation in permeabilized fibers and homogenates, an elevated oxygen regime is applied, requiring instrumental background test in the same range of elevated oxygen. |
| Oxygen kinetics | Oxygen kinetics describes the dependence of respiration of isolated mitochondria or cells on oxygen partial pressure. Frequently, a strictly hyperbolic kinetics is observed, with two parameters, the oxygen pressure at half-maximum flux, p50, and maximum flux, Jmax. The p50 is in the range of 0.2 to 0.8 kPa for cytochrome c oxidase, isolated mitochondria and small cells, strongly dependent on Jmax and coupling state. | |
| Oxygen pressure | pO2 [kPa] | Oxygen pressure or partial pressure of oxygen [kPa], related to oxygen concentration in solution by the oxygen solubility, SO2 [µM/kPa]. |
| Oxygen sensor test | POS test | The O2 sensor test is an important component of Oroboros Quality Management. The OroboPOS test is described in detail in MiPNet06.03 POS-calibration-SOP, is performed after switching on the Oroboros O2k, and is required as a basis of technical service of the instrument. |
| Oxygen signal | The oxygen signal of the Oroboros O2k is transmitted from the electrochemical polarographic oxygen sensor (OroboPOS) for each of the two O2k-chambers to DatLab. The primary signal is a current [µA] which is converted into a voltage [V] (raw signal), and calibrated in SI units for amount of substance concentration [µmol·L-1 or µM]. For technical reasons, the raw signal is given in [V] (DatLab 7 and previous) or [µA] (DatLab 8). The value of the raw signal is the same, independent of the displayed unit ([V] or [µA]). In the following sections, only [µA] is used for information on the raw signal, but the same values in [V] apply for the raw signal when using DL7 or previous versions. | |
| Oxygen solubility | SO2 [µM/kPa] | The oxygen solubility, SO2 [µM/kPa] = [(µmol·L-1)/kPa], expresses the oxygen concentration in solution in equilibrium with the oxygen pressure in a gas phase, as a function of temperature and composition of the solution. The inverse of oxygen solubility is related to the activity of dissolved oxygen. The oxygen solubility in solution, SO2(aq), depends on temperature and the concentrations of solutes in solution, whereas the dissolved oxygen concentration at equilibrium with air, cO2*(aq), depends on SO2(aq), barometric pressure and temperature. SO2(aq) in pure water is 10.56 µM/kPa at 37 °C and 12.56 µM/kPa at 25 °C. At standard barometric pressure (100 kPa), cO2*(aq) is 207.3 µM at 37 °C (19.6 kPa partial oxygen pressure) or 254.7 µM at 25 °C (20.3 kPa partial oxygen pressure). In MiR05 and serum, the corresponding saturation concentrations are lower due to the oxygen solubility factor: 191 and 184 µM at 37 °C or 234 and 227 µM at 25 °C. |
| Oxygen solubility factor | FM | The oxygen solubility factor of the incubation medium, FM, expresses the effect of the salt concentration on oxygen solubility relative to pure water. In mitochondrial respiration medium MiR05, MiR05-Kit and MiR06, FM is 0.92 (determined at 30 and 37 °C) and in culture media is 0.89 (at 37 °C). FM varies depending on the temperature and composition of the medium. To determine the FM based on the oxygen concentration, specific methods and equipment are needed (see references Rasmussen HN, Rasmussen UF 2003 in MiPNet06.03). For other media, FM may be estimated using Table 4 in MiPNet06.03. For this purpose KCl based media can be described as "seawater" of varying salinity. The original data on sucrose and KCl-media (Reynafarje et al 1985), however, have been critizesed as artefacts and the FM of 0.92 is suggested in the temperature range of 10 °C to 40 °C as for MiR05. |
| P-L control efficiency | jP-L | |
| P-L net OXPHOS capacity | P-L | |
| P/E control ratio | P/E | |
| P/O ratio | P/O ratio | P/O ratio stands for phosphate to atomic oxygen ratio, where P indicates phosphorylation of ADP to ATP (or GDP to GTP). |
| P50 | p50 | p50 is the oxygen partial pressure at which (a) respiratory flux is 50% of maximum oxygen flux, Jmax, at saturating oxygen levels. The oxygen affinity is indirectly proportional to the p50. The p50 depends on metabolic state and rate. (b) p50 is the oxygen partial pressure at which oxygen binding (on myoglobin, haemoglobin) is 50%, or desaturation is 50%. |
| PB Light Source | The PhotoBiology Light Source (PBLS) has been designed as a part of the PB-Module to provide with an external source of light. This enables experiments for evaluating the production of O2 in the presence of light. The PBLS consists of one LED and one photodiode mounted on the PBLS head protected by a PMMA plastic cover. Three pairs of PBLS (white, blue, and red) are provided with the PB-Module. The light intensity can be regulated from 0 to 2750 µmol·s-1·m-2 (red PBLS), from 0 to 3000 µmol·s-1·m-2 (blue PBLS), from 0 to 3500 µmol·s-1·m-2 (white PBLS). An integrated photodiode provides real-time measurement of the light intensity allowing for continuous adjustment to the desired value. | |
| PB-Module | The PB-Module has been developed for conducting measurements of PhotoBiology, including photosynthesis. It consists of the PB Light Source and electronic components which are an integral part of the NextGen-O2k. Measurements are recorded and evaluated with the DatLab 8 software. | |
| PBI-Shredder O2k-Set | PBI-Shredder O2k-Set: Auxiliary O2k-Tool for tissue homogenate preparation | |
| PBI-Shredder SG3 | PBI-Shredder SG3 for tissue homogenate preparation, heavy duty high torque SG3 driver with convertible handle, SG3 base with 3 position force setting lever (FSL), battery charger and two lithium ion batteries. The PBI-Shredder SG3 is included in the PBI-Shredder O2k-Set. Select 230 V or 120 V. Oroboros Instruments: world-wide distributor. | |
| PBMC | PBMC | Peripheral blood mononuclear cells (PBMC) are a fraction of the leucocyte population in the blood composed by cells with round nucleus. PBMC consist of lymphocytes (T, B and NK cells) and monocytes. During extraction, neutrophils and platelets (PLT) can be found in the PBMC fraction, where PLT are considered as a contamination. |
| PC requirements | The PC requirements for controlling an O2k and data recording with DatLab are found here. | |
| PGM-pathway control state | PGM | PGM: Pyruvate & Glutamate & Malate. MitoPathway control state: NADH electron transfer-pathway state Pyruvate (P) is oxidatively decarboxylated to acetyl-CoA and CO2, yielding NADH catalyzed by pyruvate dehydrogenase. Malate (M) is oxidized to oxaloacetate by mt-malate dehydrogenase located in the mitochondrial matrix. Condensation of oxaloacate with acetyl-CoA yields citrate (citrate synthase). Glutamate&malate is a substrate combination supporting an N-linked pathway control state, when glutamate is transported into the mt-matrix via the glutamate-aspartate carrier and reacts with oxaloacetate in the transaminase reaction to form aspartate and oxoglutarate. Glutamate as the sole substrate is transported by the electroneutral glutamate-/OH- exchanger, and is oxidized in the mitochondrial matrix by glutamate dehydrogenase to α-ketoglutarate ( 2-oxoglutarate), representing the glutamate-anaplerotic pathway control state. 2-oxoglutarate (α-ketoglutarate) is formed from isocitrate (isocitrate dehydrogenase, from oxaloacetate and glutamate by the transaminase, and from glutamate by the glutamate dehydrogenase. |