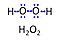Difference between revisions of "MitoPedia: Terms and abbreviations"
| Line 17: | Line 17: | ||
==References== | ==References== | ||
* Gnaiger E (1993) Nonequilibrium thermodynamics of energy transformations. Pure Appl Chem 65: 1983-2002. [[Gnaiger_1993_PAC|>>Open Access]] | * Gnaiger E (1993) Nonequilibrium thermodynamics of energy transformations. Pure Appl Chem 65: 1983-2002. [[Gnaiger_1993_PAC | >>Open Access]] | ||
* Cohen ER, Cvitas T, Frey JG, Holmström B, Kuchitsu K, Marquardt R, Mills I, Pavese F, Quack M, Stohner J, Strauss HL, Takami M, Thor HL (2008) | * Cohen ER, Cvitas T, Frey JG, Holmström B, Kuchitsu K, Marquardt R, Mills I, Pavese F, Quack M, Stohner J, Strauss HL, Takami M, Thor HL (2008) Quantities, Units and Symbols in Physical Chemistry. IUPAC Green Book, 3rd Edition, 2nd Printing, IUPAC & RSC Publishing, Cambridge. [[Cohen 2008 IUPAC Green Book | >>Open Access]] | ||
* Gnaiger E (2012) Mitochondrial Pathways and Respiratory Control. An Introduction to OXPHOS Analysis. 3rd ed. Mitochondr Physiol Network 17.18. OROBOROS MiPNet Publications, Innsbruck: 64 pp. [[Gnaiger 2012 MitoPathways|>>Open Access]] | * Gnaiger E (2012) Mitochondrial Pathways and Respiratory Control. An Introduction to OXPHOS Analysis. 3rd ed. Mitochondr Physiol Network 17.18. OROBOROS MiPNet Publications, Innsbruck: 64 pp. [[Gnaiger 2012 MitoPathways | >>Open Access]] | ||
[[Category:All]] | [[Category:All]] | ||
Revision as of 18:20, 15 April 2014
MitoPedia Glossary: Terms and abbreviations
High-resolution terminology - matching measurements at high-resolution (n.a. = no abbreviation)
The MitoPedia Glossaries are continuously developing in the spirit of Gentle Science.
| Term | Abbreviation | Description |
|---|---|---|
| % | % | The symbol % indicates 'per cent' (per hundred). {Quote} The internationally recognized symbol % (per cent) may be used with the SI. When it is used, a space separates the number and the symbol %. {end of Quote}. |
| 1OctM;2D;3PG;4S;5U;6Rot- | FNS(Oct,PGM) | 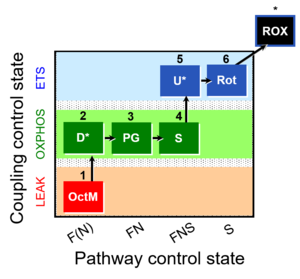 |
| 1PGM;2D;3S;4Rot;5U- | NS(PGM) | 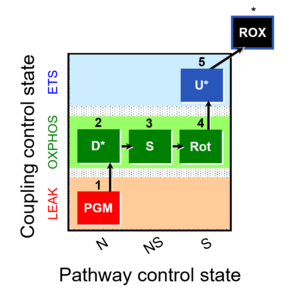 |
| 1PGM;2D;3U;4S;5Rot- | NS(PGM) | 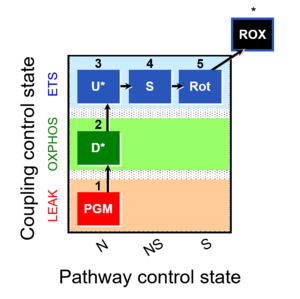 |
| 1PM;2D;3G;4U;5S;6Rot- | NS(PGM) | 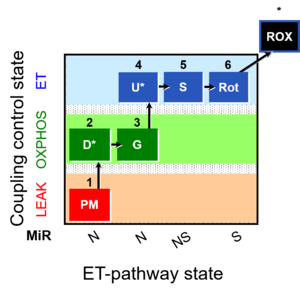 |
| 2-Deoxyglucose | 2-DG | 2-Deoxyglucose, also known as 2-deoxy-D-glucose is a glucose derivative that has the 2-hydroxyl group replaced by hydrogen. It competitively inhibits glycolysis by blocking hexokinase and phosphohexoseisomerase. |
| 2-Hydroxyglutarate | 2HG | Reduction of oxoglutarate (2OG or alpha-ketoglutarate) to 2-hydroxyglutarate (2HG) is driven by NADPH. 2HG is also formed in side reactions of lactate dehydrogenase and malate dehydrogenase. Millimolar 2HG concentrations are found in some cancer cells compared to , whereas side activities of lactate and malate dehydrogenase form submillimolar s-2-hydroxyglutarate (s-2HG). However, even wild-type IDH1 and IDH2, notably under shifts toward reductive carboxylation glutaminolysis or changes in other enzymes, lead to “intermediate” 0.01–0.1 mM 2HG levels, for example, in breast carcinoma compared with nanomolar concentrations in benign cells. 2HG is considered an important player in reprogramming metabolism of cancer cells. |
| 3-Mercaptopropionic acid | MPA | 3-Mercaptopropionic acid (MPA) inhibits long chain acyl-CoA dehydrogenases (ACADs). |
| ADP | D | Adenosine diphosphate is a nucleotide. In OXPHOS core metabolism, ADP is a substrate of ANT and ATP synthase in the phosphorylation system. ADP is the discharged or low-energy counterpart of ATP. ADP can accept chemical energy by regaining a phosphate group to become ATP, in substrate-level phosphorylation (in anaerobic catabolism), at the expense of solar energy (in photosynthetic cells) or chemiosmotic energy (respiration in heterotrophic cells). ADP is added to mitochondrial preparations at kinetically saturating concentrations to induce the active state for evaluation of OXPHOS capacity. |
| AMPK | AMPK | AMP-activated protein kinase is a regulatory protein which acts as crucial cellular energy sensor by sensing AMP, ADP and/or Ca2+ levels in response to metabolic stresses or drug administration. |
| ATP | T | Adenosine triphosphate is a nucleotid and functions as the major carrier of chemical energy in the cells. As it transfers its energy to other molecules, it looses its terminal phosphate group and becomes adenosine diphosphate (ADP). |
| ATP synthase | CV | ATP synthase or F-ATPase (F1FO-ATPase; the use of Complex V is discouraged) catalyzes the endergonic phosphorylation of ADP to ATP in an over-all exergonic process that is driven by proton translocation along the protonmotive force. The ATP synthase can be inhibited by oligomycin. |
| Abscissa | x | The abscissa is the horizontal axis x of a rectangular two-dimensional graph with the ordinate y as the vertical axis. Values X are placed horizontally from the origin. See Abscissal X/Y regression. |
| Absorbance | A | Also known as attenuation or extinction, absorbance (A) is a measure of the difference between the incident light intensity (I0) and the intensity of light emerging from a sample (I). It is defined as: A = log (I0/I) |
| Absorption | Abs | When light enters a sample and emerges with an intensity (I), absorption (Abs) is the fraction of the light absorbed by the sample compared with the incident light intensity (I0): Abs = 1-I/I0. Absorption can also be expressed as Abs = 1-T, where T is the transmittance. |
| Acceleration | a, g [m·s-2] | Acceleration, a, is the change of velocity over time [m·s-2].
a = dv/dtThe symbol g is used for acceleration of free fall. The standard acceleration of free fall is defined as gn = 9.80665 [m·s-2]. |
| Aconitase | Aco | Aconitase is a TCA cycle enzyme that catalyzes the reversible isomerization of citrate to isocitrate. Also, an isoform is also present in the cytosol acting as a trans-regulatory factor that controls iron homeostasis at a post-transcriptional level. |
| Activity | a | The activity (relative activity) is a dimensionless quantity related to the concentration or partial pressure of a dissolved substance. The activity of a dissolved substance B equals the concentration, cB [mol·L-1], at high dilution divided by the unit concentration, c° = 1 mol·L-1:
aB = cB/c° This simple relationship applies frequently to substances at high dilutions <10 mmol·L-1 (<10 mol·m-3). In general, the concentration of a solute has to be corrected for the activity coefficient (concentration basis), γB, aB = γB·cB/c° At high dilution, γB = 1. In general, the relative activity is defined by the chemical potential, µB aB = exp[(µB-µ°)/RT] |
| Acyl-CoA dehydrogenase | ACAD | Acyl-CoA dehydrogenases ACADs are localized in the mitochondrial matrix. Several ACADs are distinguished: short-chain (SCAD), medium-chain (MCAD), and long-chain (LCAD). ACAD9 is expressed in human brain. ACADs catalyze the reaction
|
| Acylcarnitine | AC | Acylcarnitines are esters derivative of carnitine and fatty acids, involved in the metabolism of fatty acids. Long-chain acylcarnitines such as palmitoylcarnitine must be transported in this form, conjugated to carnitine, into the mitochondria to deliver fatty acids for fatty acid oxidation and energy production. Medium-chain acylcarnitines such as octanoylcarnitine are also frequently used for high-resolution respirometry. |
| Additive effect of convergent electron flow | Aα&β | Additivity Aα&β describes the principle of substrate control of mitochondrial respiration with convergent electron flow. The additive effect of convergent electron flow is a consequence of electron flow converging at the Q-junction from respiratory Complexes I and II (NS or CI&II e-input). Further additivity may be observed by convergent electron flow through glycerophosphate dehydrogenase and electron-transferring flavoprotein Complex. Convergent electron flow corresponds to the operation of the TCA cycle and mitochondrial substrate supply in vivo. Physiological substrate combinations supporting convergent NS e-input are required for reconstitution of intracellular TCA cycle function. Convergent electron flow simultaneously through Complexes I and II into the Q-junction supports higher OXPHOS capacity and ET capacity than separate electron flow through either CI or CII. The convergent NS effect may be completely or partially additive, suggesting that conventional bioenergetic protocols with mt-preparations have underestimated cellular OXPHOS-capacities, due to the gating effect through a single branch. Complete additivity is defined as the condition when the sum of separately measured respiratory capacities, N + S, is identical to the capacity measured in the state with combined substrates, NS (CI&II). This condition of complete additivity, NS=N+S, would be obtained if electron channeling through supercomplex CI, CIII and CIV does not interact with the pool of redox intermediates in the pathway from CII to CIII and CIV, and if the capacity of the phosphorylation system does not limit OXPHOS capacity (excess E-P capacity factor is zero). In most cases, however, additivity is incomplete, NS < N+S. |
| Adenine nucleotide translocase | ANT | The adenine nucleotide translocator, ANT, exchanges ADP for ATP in an electrogenic antiport across the inner mt-membrane. The ANT is inhibited by atractyloside, carboxyatractyloside and bongkrekik acid. The ANT is a component of the phosphorylation system. |
| Adenine nucleotides | AN | Adenine nucleotides, which are also sometimes referred to as adenosines or adenylates, are a group of organic molecules including AMP, ADP and ATP. These molecules present the major players of energy storage and transfer. |
| Adenylate kinase | ADK | Adenylate kinase, which is also called myokinase, is a phosphotransferase enzyme that is located in the mitochondrial intermembrane space and catalyzes the rephosphorylation of AMP to ADP in the reaction ATP + AMP ↔ ADP + ADP. |
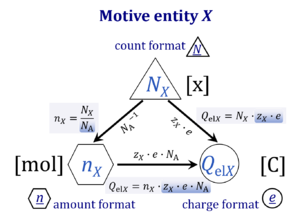
| Advancement | dtrξ [MU] | In an isomorphic analysis, any form of flow is the advancement of a process per unit of time, expressed in a specific motive unit [MU∙s-1], e.g., ampere for electric flow or current, Iel = delξ/dt [A≡C∙s-1], watt for thermal or heat flow, Ith = dthξ/dt [W≡J∙s-1], and for chemical flow of reaction, Ir = drξ/dt, the unit is [mol∙s-1] (extent of reaction per time). The corresponding motive forces are the partial exergy (Gibbs energy) changes per advancement [J∙MU-1], expressed in volt for electric force, ΔelF = ∂G/∂elξ [V≡J∙C-1], dimensionless for thermal force, ΔthF = ∂G/∂thξ [J∙J-1], and for chemical force, ΔrF = ∂G/∂rξ, the unit is [J∙mol-1], which deserves a specific acronym [Jol] comparable to volt [V]. For chemical processes of reaction (spontaneous from high-potential substrates to low-potential products) and compartmental diffusion (spontaneous from a high-potential compartment to a low-potential compartment), the advancement is the amount of motive substance that has undergone a compartmental transformation [mol]. The concept was originally introduced by De Donder [1]. Central to the concept of advancement is the stoichiometric number, νi, associated with each motive component i (transformant [2]).
In a chemical reaction r the motive entity is the stoichiometric amount of reactant, drni, with stoichiometric number νi. The advancement of the chemical reaction, drξ [mol], is defined as, drξ = drni·νi-1 The flow of the chemical reaction, Ir [mol·s-1], is advancement per time, Ir = drξ·dt-1 This concept of advancement is extended to compartmental diffusion and the advancement of charged particles [3], and to any discontinuous transformation in compartmental systems [2], |
| Advancement per volume | dtrY [MU∙L-1] | Advancement per volume or volume-specific advancement, dtrY, is related to advancement of a transformation, dtrY = dtrξ∙V-1 [MU∙L-1]. Compare dtrY with the amount of substance j per volume, cj (concentration), related to amount, cj = nj∙V-1 [mol∙V-1]. Advancement per volume is particularly introduced for chemical reactions, drY, and has the dimension of concentration (amount per volume [mol∙L-1]). In an open system at steady-state, however, the concentration does not change as the reaction advances. Only in closed systems and isolated systems, specific advancement equals the change in concentration divided by the stoichiometric number, drY = dcj/νj (closed system) drY = drcj/νj (general) With a focus on internal transformations (i; specifically: chemical reactions, r), dcj is replaced by the partial change of concentration, drcj (a transformation variable or process variable). drcj contributes to the total change of concentration, dcj (a system variable or variable of state). In open systems at steady-state, drcj is compensated by external processes, decj = -drcj, exerting an effect on the total concentration change of substance j, dcj = drcj + decj = 0 (steady state) dcj = drcj + decj (general) |
| Aerobic | ox | The aerobic state of metabolism is defined by the presence of oxygen (air) and therefore the potential for oxidative reactions (ox) to proceed, particularly in oxidative phosphorylation (OXPHOS). Aerobic metabolism (with involvement of oxygen) is contrasted with anaerobic metabolism (without involvement of oxygen): Whereas anaerobic metabolism may proceed in the absence or presence of oxygen (anoxic or oxic conditions), aerobic metabolism is restricted to oxic conditions. Below the critical oxygen pressure, aerobic ATP production decreases. |
| Affinity of reaction | A [J·mol-1] | The concept of affinity and hence chemical force is deeply rooted in the notion of attraction (and repulsion) of alchemy, which was the foundation of chemistry originally, but diverted away from laboratory experiments towards occult secret societies [1].** Newton's extensive experimental alchemical work and his substantial written track record on alchemy (which he did not publish) is seen today as a key inspiration for his development of the concept of the gravitational force [2-4]. This marks a transition of the meaning of affinity, from the descriptive 'adjacent' (proximity) to the causative 'attractive' (force) [5]. Correspondingly, Lavoisier (1790) equates affinity and force [6]: “... the degree of force or affinity with which the acid adheres to the base” [5]. By discussing the influence of electricity and gravity on chemical affinity, Liebig (1844) considers affinity as a force [7]. This leads to Guldberg and Waage's mass action ratio ('Studies concerning affinity', 1864; see [5]), the free energy and chemical affinity of Helmholtz (1882 [8]), and chemical thermodynamics of irreversible processes [9], where flux-force relations are center stage [10].
According to the IUPAC definition, the affinity of reaction, A [J·mol-1], equals the negative molar Gibbs energy of reaction [11], which is the negative Gibbs force of reaction (derivative of Gibbs energy per advancement of reaction [12]): -A = ΔrF = ∂G/∂rξThe historical account of affinity is summarized by concluding, that today affinity of reaction should be considered as an isomorphic motive force and be generalized as such. This will help to (1) avoid confusing reversals of sign conventions (repulsion = negative attraction; pull = negative push), (2) unify symbols across classical and nonequilibrium thermodynamics [12,13], and thus (3) facilitate interdisciplinary communication by freeing ourselves from the alchemical, arcane scientific nomenclature. |
| Air calibration | R1 | Air calibration of an oxygen sensor (polarographic oxygen sensor) is performed routinely on any day before starting a respirometric experiment. The volume fraction of oxygen in dry air is constant. An aqueous solution in equilibrium with air has the same partial pressure as that in water vapour saturated air. The water vapour is a function of temperature only. The partial oxygen pressure in aqueous solution in equilibrium with air is, therefore, a function of total barometric pressure and temperature. Bubbling an aqueous solution with air generates deviations from barometric pressure within small gas bubbles and is, therefore, not recommended. To equilibrate an aqueous solution ata known partial pressure of oxygen [kPa], the aqueous solution is stirred rigorously in a chamber enclosing air at constant temperature. The concentration of oxygen, cO2 [µM], is obtained at any partial pressure by multiplying the partial pressure by the oxygen solubility, SO2 [µM/kPa]. SO2 is a function of temperature and composition of the salt solution, and is thus a function of the experimental medium. The solubility factor of the medium, FM, expresses the oxygen solubility relative to pure water at any experimental temperature. FM is 0.89 in serum (37 °C) and 0.92 in MiR06 or MiR05 (30 °C and 37 °C). |
| Alternative oxidase | AOX | Alternative quinol oxidases AOX are membrane-bound enzymes capable of supporting cyanide- and antimycin A-resistant mitochondrial respiration. AOX catalyzes the oxidation of ubiquinol and the reduction of oxygen to water in a four-electron process. As this bypasses several proton-translocating steps, induction of this alternative pathway is associated with a reduction of ATP production per oxygen consumed. AOX is found in most plants (including microalgae), many fungi and protists, but is not expressed in animals. AOX is inhibited by salicylhydroxamic acid (SHAM). Expression and activity of the enzyme are modified by environmental conditions such as temperature, oxidative stress, nutrient availability, and pathogens such as viruses. |
| Ammonia solution concentrated | NH3 | Concentrated ammonia solution (25 % - 30 % ammonium hydroxide solution, ammonia) is used for the service of the polarographic oxygen sensor OroboPOS. After opening the commercial solution, the concentration of ammonia may decline during storage and may render the ammonia stock ineffective for sensor service. Source: A commercially available solution from a drugstore is sufficient for this cleaning purpose |
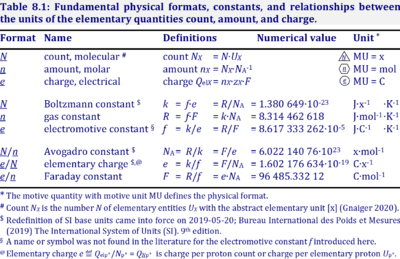
| Amount of substance | n [mol] | The amount of substance n is a base physical quantity, and the corresponding SI unit is the mole [mol]. Amount of substance (sometimes abbreviated as 'amount' or 'chemical amount') is proportional to the number NX of specified elementary entities X, and the universal proportionality constant is the reciprocal value of the Avogadro constant (SI),
nX = NX·NA-1 nX contained in a system can change due to internal and external transformations, dnX = dinX + denX In the absence of nuclear reactions, the amount of any atom is conserved, e.g., for carbon dinC = 0. This is different for chemical substances or ionic species which are produced or consumed during the advancement of a reaction r, A change in the amount of Xi, dni, in an open system is due to both the internal formation in chemical transformations, drni, and the external transfer, deni, across the system boundaries. dni is positive if Xi is formed as a product of the reaction within the system. deni is negative if Xi flows out of the system and appears as a product in the surroundings (Cohen 2008 IUPAC Green Book). |
| Amp calibration - DatLab | F5 | Amp calibration indicates the calibration of the amperometric O2k-channel. |
| Ampere | A | The ampere, symbol A, is the SI unit of electric current. It is defined by taking the fixed numerical value of the elementary charge e to be 1.602 176 634 × 10−19 when expressed in the unit C, which is equal to A s, where the second is defined in terms of ΔνCs. |
| Amperometric,Amp | F7 | After selection of the Amperometric, Amp channel in the O2k configuration, an Amperometric, Amp tab will appear in the O2k control [F7] window. Set the desired light intensity (0-1600) in the field ´Fluo intensity´ and the desired amplification of the signal (1-1000) in the field ´Gain for Fluo sensor´in the Amperometric, Amp window followed by a left-click Send to O2k. Switching off the illumination before each fluorometric measurement is routinely required. |
| Amplex UltraRed | AmR | Amplex® UltraRed (AmR) is used as an extrinsic fluorophore for measurement of hydrogen peroxide production (ROS) by cells or mitochondrial preparations. The reaction of H2O2 and AmR is catalyzed by horseradish peroxidase to produce the red fluorescent compound resorufin (excitation wavelength 563 nm, emission 587 nm; the fluorescent product according to the supplier is called UltroxRed in the case of Amplex® UltraRed which has a similar structure to resorufin). The change of emitted fluorescence intensity is directly proportional to the concentration of H2O2 added, whereby the H2O2 is consumed. |
| Amytal | Amy | Amytal sodium salt (synonym: amobarbital; 5-Ethyl-5-isoamylbarbituric acid) is a barbiturate drug and an inhibitor of Complex I. |
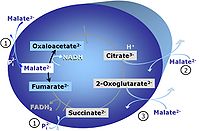
| Anaplerotic pathway control state | a | Anaplerotic pathway control states are fuelled by single substrates which are transported into the mitochondrial matrix and increase the pool of intermediates of the tricarboxylic acid cycle. Malic enzyme (mtME), phosphoenopyruvate carboxykinase (PEPCK), propionyl-CoA carboxylase, and pyruvate carboxylase play important roles in anaplerosis. The glutamate-anaplerotic pathway control state and malate-anaplerotic pathway control state are the most important anaplerotic substrate control states (aN). |
| Anoxia | anox | Ideally the terms anoxia and anoxic (anox, without oxygen) should be restricted to conditions where molecular oxygen is strictly absent. Practically, effective anoxia is obtained when a further decrease of experimental oxygen levels does not elicit any physiological or biochemical response. The practical definition, therefore, depends on (i) the techiques applied for oxygen removal and minimizing oxygen diffusion into the experimental system, (ii) the sensitivity and limit of detection of analytical methods of measuring oxygen (O2 concentration in the nM range), and (iii) the types of diagnostic tests applied to evaluate effects of trace amounts of oxygen on physiological and biochemical processes. The difficulties involved in defining an absolute limit between anoxic and microxic conditions are best illustrated by a logarithmic scale of oxygen pressure or oxygen concentration. In the anoxic state (State 5), any aerobic type of metabolism cannot take place, whereas anaerobic metabolism may proceed under oxic or anoxic conditions. |
| Antimycin A | Ama | Antimycin A is an inhibitor of Complex III (CIII). It binds to the Qi site of CIII and inhibits the transfer of electrons from heme bH to oxidized Q (Qi site inhibitor). High concentrations of antimycin A also inhibit acyl-CoA oxidase and D-amino acid oxidase. |
| Ap5A | Ap5A | P1,P5-Di(adenosine-5')pentaphosphate (Ap5A) is an inhibitor of adenylate kinase (ADK), the enzyme which rephosphorylates AMP to ADP, consuming ATP (ATP + AMP ↔ 2 ADP). |
| Aqua destillata | a.d. | Aqua destillata (a.d.) is the Latin name for distilled water, H2O. When a.d. is used in various solution protocols, it may indicate that water with the highest possible quality or lowest possible level of impurities should be used, as may be reached not only with distilled water but also with high-purity deionised water. |
| ArXiv preprint server | arXiv | arXiv is a classic preprint server initiated in 1991 by Paul Ginsparg. {Quote} arXiv.org is a highly-automated electronic archive and distribution server for research articles. Covered areas include physics, mathematics, computer science, nonlinear sciences, quantitative biology, quantitative finance, statistics, electrical engineering and systems science, and economics. arXiv is maintained and operated by Cornell University with guidance from the arXiv Scientific Advisory Board and the arXiv Member Advisory Board, and with the help of numerous subject moderators. {end of Quote}. arXiv rejects abstracts that are submitted without accompanying paper. |
| Ascorbate | As | In respiratory assays for cytochrome c oxidase activity (Complex IV, CIV), ascorbate is added as regenerating system to maintain TMPD in a reduced state. It has to be titrated into the respiration medium prior to the addition of TMPD, otherwise the autoxidation reaction velocity is permanently elevated. |
| Asia Society for Mitochondrial Research and Medicine | ASMRM | The Asia Society for Mitochondrial Research and Medicine (ASMRM) was founded in 2003 to share the latest knowledge on mitochondrial research. |
| Atractyloside | Atr | Atractyloside is an inhibitor of the adenine nucleotide translocator (ANT). It is an extremely toxic glycoside that inhibits oxidative phosphorylation by blocking the transfer of adenosine nucleotides through the mitochondrial membrane. |
| Auranofin | AF | Auranofin (AF) is a gold complex which inhibites thioredoxin reductase (TrxR). |
| Avogadro constant | NA [x·mol-1] | {Quote} The Avogadro constant NA is a proportionality constant between the quantity amount of substance (with unit mole) and the quantity for counting entities ... One mole contains exactly 6.022 140 76 × 1023 elementary entities. This number is the fixed numerical value of the Avogadro constant, NA, when expressed in the unit mol−1 and is called the Avogadro number {End of Quote: Bureau International des Poids et Mesures 2019 The International System of Units (SI)}. Thus the Avogadro constant NA has the SI unit 'per mole' [mol-1], but more strictly the unit for counting entities per amount is 'units per mole' [x·mol-1] (compare elementary charge). Therefore, NA is 'count per amount' with units 'counting units per mole'. The Avogadro constant times elementary charge is the Faraday constant. |
| Azide | Azd | Sodium azide is an inhibitor of Complex IV/cytochrome c oxidase (CIV, COX, CcO). |
| BAM15 | BAM15 | 2-fluorophenyl){6-[(2-fluorophenyl)amino](1,2,5-oxadiazolo[3,4-e]pyrazin-5-yl)}amine (BAM15) is a protonophore or uncoupler of oxidative phosphorylation detected in a screen for uncoupling agents exerting less toxicity than commonly used uncouplers and first described by Kennwood et al. 2013. In their comparison of BAM15 with FCCP it was shown to increase oxygen flux to a similar extent as the classical uncoupler, to display a much broader range of concentrations inducing maximum respiration, to stimulate no formation of H2O2, to leave cellular membrane potential unaffected, and to ultimately exert less cytotoxicity. |
| BME cutoff points | BME cutoff | Obesity is defined as a disease associated with an excess of body fat with respect to a healthy reference condition. Cutoff points for body mass excess, BME cutoff points, define the critical values for underweight (-0.1 and -0.2), overweight (0.2), and various degrees of obesity (0.4, 0.6, 0.8, and above). BME cutoffs are calibrated by crossover-points of BME with established BMI cutoffs. |
| Background state | Y | The background state Y (background rate YX) is the non-activated or inhibited respiratory state at background rate, which is low in relation to the higher rate ZX in the reference state Z. The transition from the background state to the reference state is a step change. A metabolic control variable X (substrate, activator) is added to the background state to stimulate flux to the level of the reference state. Alternatively, the metabolic control variable X is an inhibitor, which is present in the background state Y, but absent in the reference state Z. The background state is the baseline of a single step in the definition of the flux control efficiency. In a sequence of step changes, the common baseline state is the state of lowest flux in relation to all steps, which can be used as a baseline correction. |
| Barometric pressure | pb [Pa] | Barometric pressure, pb, is an important variable measured for calibration of oxygen sensors in solutions equilibrated with air. The atm-standard pressure (1 atm = 760 mmHg = 101.325 kPa) has been replaced by the SI standard pressure of 100 kPa. The partial pressure of oxygen, pO2, in air is a function of barometric pressure, which changes with altitude and locally with weather conditions. The partial oxygen pressure declines by 12 % to 14 % per 1,000 m up to 6,000 m altitude, and by 15 % to 17 % per 1,000 m between 6,000 and 9,000 m altitude. The O2k-Barometric Pressure Transducer is built into the Oroboros O2k as a basis for accurate air calibrations in high-resolution respirometry. For highest-level accuracy of calculation of oxygen pressure, it is recommended to compare at regular intervals the barometric pressure recording provided by the O2k with a calibrated barometric pressure recording at an identical time point and identical altitude. The concept of gas pressure or barometric pressure can be related to the generalized concept of isomorphic pressure. |
| Barth Syndome | BTHS | Barth Syndome (BTHS) is an X-linked genetic condition that is caused by a mutation in the tafazzin gene (taz). This mutation causes cardiolipin abnormalities, cardiomyopathy, neutropenia, muscle weakness, growth delay, and exercise intolerance. Contributed by Sparagna GC 2016-04-24 |
| Basal respiration | BMR | Basal respiration or basal metabolic rate (BMR) is the minimal rate of metabolism required to support basic body functions, essential for maintenance only. BMR (in humans) is measured at rest 12 to 14 hours after eating in a physically and mentally relaxed state at thermally neutral room temperature. Maintenance energy requirements include mainly the metabolic costs of protein turnover and ion homeostasis. In many aerobic organisms, and particularly well studied in mammals, BMR is fully aerobic, i.e. direct calorimetry (measurement of heat dissipation) and indirect calorimetry (measurement of oxygen consumption multiplied by the oxycaloric equivalent) agree within errors of measurement (Blaxter KL 1962. The energy metabolism of ruminants. Hutchinson, London: 332 pp [1]). In many cultured mammalian cells, aerobic glycolysis contributes to total ATP turnover (Gnaiger and Kemp 1990 [2]), and under these conditions, 'respiration' is not equivalent to 'metabolic rate'. Basal respiration in humans and skeletal muscle mitochondrial function (oxygen kinetics) are correlated (Larsen et al 2011 [3]). » MiPNet article |
| Beer-Lambert law | B-L law | This law states that the transmittance (T) of light though a sample is given by: T = e-εbc, where ε is the molar extinction coefficient, b is the pathlength of the light through the cuvette (in mm) and c is the concentration of the pigment in the sample (in mM). Transforming this equation, it can be seen that the absorbance of light (A) is simply given by A = εbc. |
| Beryllium sulfate | BeS | Beryllium sulfate is used in combination with sodium fluoride to form beryllium trifluoride (BeF3−), to inhibit the ATP synthase if it is exposed by disruption of the mitochondrial membranes. |
| BioRxiv preprint server for biology | bioRxiv | bioRxiv (pronounced "bio-archive") is a free online archive and distribution service for unpublished preprints in the life sciences. It was launched in 2013 by Cold Spring Harbor Laboratory Press in New York, and is operated by Cold Spring Harbor Laboratory, a not-for-profit research and educational institution. By posting preprints on bioRxiv, authors are able to make their findings immediately available to the scientific community and receive feedback on draft manuscripts before they are submitted to journals. bioRxiv is intended for rapid sharing of new research. Some review articles contain new data/analyses and may therefore be deemed appropriate. Reviews that solely summarize existing knowledge are not appropriate and neither are term papers, book excerpts, and undergraduate dissertations. |
| Bioblasts | BB | Richard Altmann (1894) defined the 'elementary organisms' as Bioblasts. He observed granula in cells stained with osmium and viewed ‘the protoplasm as a colony of bioblasts’. "Microorganisms and granula are at an equivalent level and represent elementary organisms, which are found wherever living forces are acting, thus we want to describe them by the common term bioblasts. In the bioblast, that morphological unit of living matter appears to be found." Altmann 1894; p. 141. Altmann is thus considered as the discoverer of mitochondria (the granula), which constitute together with the microorganisms the bioblasts (the elementary organisms). Bioblasts are the aliens with permanent residence in our cells (Gnaiger 2010). |
| Biochemical coupling efficiency | jE-L | |
| Biopsy preservation solution | BIOPS | Biopsy preservation solution, for preservation of tissue samples, preparation of muscle fibres, and permeabilization with saponin. |
| Blebbistatin | Bleb | Blebbistatin is a widely used muscle and non-muscle myosin II-specific inhibitor that block contractile activity. Blebbistatin shows selectivity and high affinity for multiple class II myosins. Blebbistatin is commonly employed in respirometric experiments with permeabilized muscle fibers (pfi). Permeabilized muscle fibers are sensitive to low oxygen supply due to diffusion restrictions that limit mitochondrial respiration at the core of the fiber bundle. Therefore, hyperoxic conditions are required to counteract this limitation. Further studies have shown that the addition of blebbistatin in the respiration medium prevents fiber contraction, reduces the oxygen sensitivity and allows the study of ADP kinetics in pfi at normoxic oxygen levels. However, other studies described that the presence of blebbistatin does not prevent the oxygen dependence in pfi. Moreover, several limitations of blebbistatin i.e. low solubility in water, cytotoxicity and phototoxicity have been described. |
| Blood cell preparation | bcp | Blood cell preparation (bcp) is one of the key steps in diagnostic protocols. |
| Blood plasma | Plasma | Blood plasma is the non-cellular component of the blood. Plasma lacks cellular components of the blood, red blood cells, white blood cells, and platelets. However, there are many proteins in plasma, i.e. fibrinogen, albumin and globulin. Both blood plasma and platelet-rich plasma maintain clotting activity after whole blood separation. |
| Blood serum | Serum | Blood serum is a purified plasma in which the coagulant components were removed from the blood plasma. It contains other substances, i.e. antibodies, antigens and hormones. Serum can be obtained by collecting the liquid phase after blood or plasma coagulation. |
| Body fat excess | BFE | In the healthy reference population (HRP), there is zero body fat excess, BFE, and the fraction of excess body fat in the HRP is expressed - by definition - relative to the reference body mass, M°, at any given height. Importantly, body fat excess, BFE, and body mass excess, BME, are linearly related, which is not the case for the body mass index, BMI. |
| Body mass | m [kg]; M [kg·x-1] | The body mass M is the mass (kilogram [kg]) of an individual (object) [x] and is expressed in units [kg/x]. Whereas the body weight changes as a function of gravitational force (you are weightless at zero gravity; your floating weight in water is different from your weight in air), your mass is independent of gravitational force, and it is the same in air and water. |
| Body mass excess | BME | The body mass excess, BME, is an index of obesity and as such BME is a lifestyle metric. The BME is a measure of the extent to which your actual body mass, M [kg/x], deviates from M° [kg/x], which is the reference body mass [kg] per individual [x] without excess body fat in the healthy reference population, HRP. A balanced BME is BME° = 0.0 with a band width of -0.1 towards underweight and +0.2 towards overweight. The BME is linearly related to the body fat excess. |
| Body mass index | BMI | The body mass index, BMI, is the ratio of body mass to height squared (BMI=M·H-2), recommended by the WHO as a general indicator of underweight (BMI<18.5 kg·m-2), overweight (BMI>25 kg·m-2) and obesity (BMI>30 kg·m-2). Keys et al (1972; see 2014) emphasized that 'the prime criterion must be the relative independence of the index from height'. It is exactly the dependence of the BMI on height - from children to adults, women to men, Caucasians to Asians -, which requires adjustments of BMI-cutoff points. This deficiency is resolved by the body mass excess relative to the healthy reference population. |
| Boltzmann constant | k [J·x-1·K-1] | The Boltzmann constant k has the SI unit [J·K-1] (IUPAC), but more strictly the units for energy per particles per temperature is [J·x-1·K-1].
k = f·e-1, the electrochemical constant f times the elementary charge e. k = R·NA-1, the gas constant R divided by the Avogadro constant NA. |
| Bongkrekik acid | Bka | Bongkrekik acid is a selective and potent inhibitor of the adenine nucleotide translocator (ANT). Bka binds to the matrix (negative) site of ANT, opposite of carboxyatractyloside. |
| Bound energy | B [J] | The bound energy change in a closed system is that part of the total energy change that is always bound to an exchange of heat,
dB = dU - dA [Eq. 1] ∆B = ∆H - ∆G [Eq. 2] The free energy change (Helmoltz or Gibbs; dA or dG) is the total energy change (total inner energy or enthalpy, dU or dH) of a system minus the bound energy change. Therefore, if a process occurs at equilibrium, when dG = 0 (at constant gas pressure), then dH = dB, and at deW = 0 (dH = deQ + deW; see energy) we obtain the definition of the bound energy as the heat change taking place in an equilibrium process (eq), dB = T∙dS = deQeq [Eq. 3] |
| Bovine serum albumin | BSA | Bovine serum albumin is a membrane stabilizer, oxygen radical scavenger, and binds Ca2+ and free fatty acids, hence the rather expensive essentially free fatty acid free BSA is required in mitochondrial isolation and respiration media. Sigma A 6003 fraction V. |
| Buffer Z | Buffer Z | Mitochondrial respiration medium, Buffer Z, described by Perry 2011 Biochem J For composition and comparison see: Mitochondrial respiration media: comparison |
| CDGSH iron-sulfur domain proteins | CISD proteins | The CDGSH iron-sulfur domain (CISDs) family of proteins uniquely ligate labile 2Fe-2S clusters with a 3Cys-1His motif. CISD1 and CISD3 have been demonstrated to localize to the outer mitochondrial membrane and mitochondrial matrix respectively, however their relationship to mitochondrial physiology remains ill-defined [1]. The best characterized member of the CISD family, CISD1, has been demonstrated to be involved in respiratory capacity, iron homeostasis, and ROS regulation |
| CE | CE | CE marking is a mandatory conformity marking for certain products sold within the European Economic Area (EEA). |
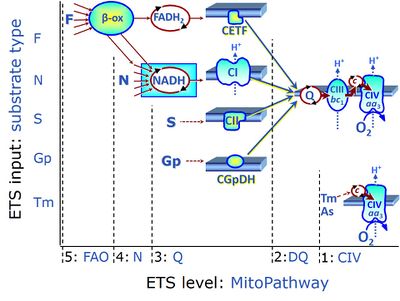
| CHNO-fuel substrate | CHNO | CHNO-fuel substrates are reduced carbon-hydrogen-nitrogen-oxygen substrates which are oxidized in the exergonic process of cell respiration. Mitochondrial pathways are stimulated by CHNO-fuel substrates feeding electrons into the ETS at different levels of integration and in the presence or absence of inhibitors acting on specific enzymes which are gate-keepers and control various pathway segments. |
| CI control ratio | N/NS; CI/CI&II | See N/NS pathway control ratio |
| CII control ratio | S/NS; CII/CI&II | See S/NS pathway control ratio |
| Calcium | Ca | Ca2+ is a major signaling molecule in both prokaryotes and eukaryotes. Its cytoplasmic concentration is tightly regulated by transporters in the plasma membrane and in the membranes of various organelles. For this purpose, it is either extruded from the cell through exchangers and pumps or stored in organelles such as the endoplasmic reticulum and the mitochondria. Changes in the concentration of the cation regulate numerous enzymes including many involved in ATP utilizing and in ATP generating pathways and thus ultimately control metabolic activity of mitochondria and of the entire cell. Measuring changes in Ca2+ levels is thus of considerable interest in the context of high-resolution respirometry. |
| Calcium Green | CaG | Calcium GreenTM (CaG) denotes a family of extrinsic fluorophores applied for measurement of Ca2+ concentration with mitochondrial preparations. This dye fluoresces when bound to Ca2+. When measuring mitochondrial calcium uptake it is possible to observe the increase of the CaG signal upon calcium titration, followed by the decrease of CaG signal due to the uptake. |
| Calcium retention capacity | CaRC | Calcium retention capacity (CaRC) is a measure of the capability of mitochondria to retain calcium (Ca2+), primarily in the form of calcium phosphates, in the mitochondrial matrix. By storing calcium in the form of osmotically inactive precipitates the mitochondria contribute to the buffering of cytosolic free Ca2+ levels and thereby to the regulation of calcium-dependent cellular processes. Alterations of CaRC are important in stress phenomena associated with energy limitation and have been linked to neurodegenerative diseases (Starkov 2013 FEBS J). Experimentally, CaRC has been indirectly assessed by determination of respiratory rates of isolated mitochondria which were exposed to continuously increasing doses of Ca2+ by use of the Titration-Injection microPump TIP2k. The upper limit of CaRC was observed as a sudden decrease of respiration presumed to reflect opening of the permeability transition pore (Hansson 2010 J Biol Chem). |
| Calorespirometric ratio | CR ratio [kJ/mol] | The calorimetric/respirometric or calorespirometric ratio (CR ratio) is the ratio of calorimetrically and respirometrically measured heat and oxygen flux, determinded by calorespirometry. The experimental CR ratio is compared with the theoretically derived oxycaloric equivalent, and agreement in the range of -450 to -480 kJ/mol O2 indicates a balanced aerobic energy budget (Gnaiger and Staudigl 1987). In the transition from aerobic to anaerobic metabolism, there is a limiting pO2, plim, below which CR ratios become more exothermic since anaerobic energy flux is switched on. |
| Calorespirometry | CR | Calorespirometry is the method of measuring simultaneously metabolic heat flux (calorimetry) and oxygen flux (respirometry). The calorespirometric ratio (CR ratio; heat/oxygen flux ratio) is thus experimentally determined and can be compared with the theoretical oxycaloric equivalent, as a test of the aerobic energy balance. |
| Candela | cd | The candela, symbol cd, is the SI unit of luminous intensity in a given direction. It is defined by taking the fixed numerical value of the luminous efficacy of monochromatic radiation of frequency 540 × 1012 Hz, Kcd, to be 683 when expressed in the unit lm W−1. |
| Carbonyl cyanide m-chlorophenyl hydrazone | CCCP | Carbonyl cyanide m-chlorophenyl hydrazone, CCCP (U; C9H5ClN4; FW = 204.62) is a protonophore (H+ ionophore) and is used as a potent chemical uncoupler of oxidative phosphorylation. Like all uncouplers, CCCP concentrations must be titrated carefully to evaluated the optimum concentration for maximum stimulation of mitochondrial respiration, particularly to avoid inhibition of respiration at higher CCCP concentrations. |
| Carboxy SNARF 1 | SNARF | Carboxy SNARF® 1 is a cell-impermeant pH indicator dye. The pKa of ~7.5 makes it useful for measuring pH in the range of pH 7 to pH 8. The emission shifts from yellow-orange at low pH to deep red fluorescence at high pH. Ratiometric fluorometry, therefore, is applied at two emission wavelengths,such as 580 nm and 640 nm. Relative molecular mass: Mr = 453.45 |
| Carboxyatractyloside | CAT | Carboxyatractyloside CAT is a highly selective and potent inhibitor of the adenine nucleotide translocator (ANT). CAT stabilizes the nucleoside binding site of ANT on the cytoplasmic (positive) side of the inner membrane and blocks the exchange of matrix ATP and cytoplasmic ADP. It causes stabilization of the c conformation of ANT leading to permeability transition pore (PTP) opening, loss of mitochondrial membrane potential, and apoptosis. |
| Cardiolipin | CL | Cardiolipin, CL, is a double phospholipid (having 4 fatty acyl chains) in the mitochondrial inner membrane (mtIM) which plays an important role in mitochondrial bioenergetics. CL is involved in the mitochondria-dependent pathway of apoptosis, participates in the function and stabilization of mitochondrial respiratory complexes and supercomplexes and also contributes to mitochondrial integrity. Contributed by Sparagna G 2016-04-18 |
| Cardiovascular Exercise Research Group | CERG |
The Cardiovascular Exercise Research Group (CERG) was established in January 2008 and their research focuses on identifying the key cellular and molecular mechanisms underlying the beneficial effects of physical exercise on the heart, arteries and skeletal muscle in the context of disease prevention and management through experimental, clinical and epidemiological studies. Since 2003 this research group organizes the biennial seminar "Exercise in Medicine" in Trondheim, Norway. |
| Carnitine | Car | Carnitine is an important factor for the transport of long-chain fatty acids bound to carnitine (carnitine acyltransferase) into the mitochondrial matrix for subsequent β-oxidation. There are two enantiomers: D- and L-carnitine. Only the L-isomer is physiologically active. |
| Carnitine O-octanoyltransferase | COT | Carnitine O-octanoyltransferase is a mitochondrial enzyme that transfers carnitine to octanoyl-CoA to form Coenzyme A and octanoylcarnitine: Octanoyl-CoA + L-carnitine ↔ CoA + L-octanoylcarnitine. |
| Carnitine acetyltransferase | CrAT | Carnitine acetyltransferase (CrAT) is located in the mitochondrial matrix and catalyses the formation of acetyl-carnitine from acetyl-CoA and L-carnitine and thus regulates the acetyl-CoA/free CoA ratio which is essential for pyruvate dehydrogenase complex (PDC) activity. |
| Carnitine palmitoyltransferase I | CPT-I | Carnitine palmitoyltransferase I (CPT-I, also known as carnitine acyltransferase I) is a regulatory enzyme in mitochondrial long-chain acyl-CoA uptake and further oxidation. CPT-I is associated with the mt-outer membrane mtOM and catalyses the formation of acylcarnitines from acyl-CoA and L-carnitine. In the next step, acyl-carnitines are transported to the mitochondrial matrix via carnitine-acylcarnitine translocase in exchange for free carnitine. In the inner side of the mtIM carnitine palmitoyltransferase II converts the acyl-carnitines to carnitine and acyl-CoAs. There are three enzyme isoforms: CPT-1A (liver type), CPT-1B (muscle type), CPT-1C (brain type). Isoforms have significantly different kinetic and regulatory properties. Malonyl-CoA is an endogenous inhibitor of CPT-I. |
| Carnitine palmitoyltransferase II | CPT-II | Carnitine palmitoyltransferase II (CPT-II, also known as carnitine acyltransferase II) is part of the carnitine shuttle which is responsible for the mitochondrial transport of long-chain fatty acids. CPT-II is located on the inner side of the mtIM and converts the acylcarnitines (produced in the reaction catalyzed by carnitine palmitoyltransferase I) to carnitine and acyl-CoAs, which undergo ß-oxidation in the mitochondrial matrix. Free carnitines are transported out of the mitochondrial matrix in exchange for acyl-carnitines via an integral mtIM protein carnitine-acylcarnitine translocase (CACT). Short- and medium-chain fatty acids do not require the carnitine shuttle for mitochondrial transport. |
| Carnitine-acylcarnitine translocase | CACT | Carnitine-acylcarnitine translocase (CACT) is part of the carnitine shuttle which mediates the mitochondrial transport of long-chain fatty acids where the fatty acid oxidation occurs. CACT is an internal mt-IM protein and transports acylcarnitines into the mitochondrial matrix in exchange for free carnitine. |
| Catalase | Ctl | Catalase catalyzes the dismutation of hydrogen peroxide to water and oxygen. Perhaps all cells have catalase, but mitochondria of most cells lack catalase. Cardiac mitochondria are exceptional in having mt-catalase activity (rat heart mitochondria: Radi et al 1991; mouse heart mitochondria: Rindler et al 2013). Hydroxylamine is an inhibitor of catalase, which is also inhibited by cyanide and azide. Mitochondrial respiration medium MiR05 was developed considering the intracellular conditions of mitochondria in living cells. In mitochondrial preparations, enzymes and substrates present in the cytosol (such as catalase) are diluted when the plasma membrane is removed. Therefore, the addition of catalase is recommended when working with mitochondrial preparations, to consume any H2O2 generated during the assay. |
| Catalytic activity | kat | Catalytic activity of an enzyme is measured by an enzyme assay and is expressed in units of katal (kat [mol∙s-1]). More commonly (but not conforming to SI units or IUPAC recommendations) enzyme activity is expressed in units U [mol∙min-1]. |
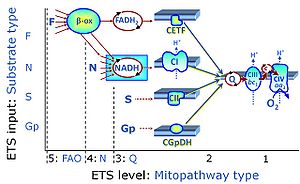
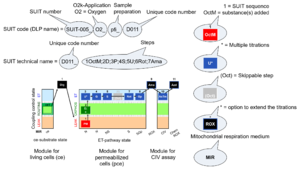
| Categories of SUIT protocols | SUIT-catg |
Categories of SUIT protocols group SUIT protocols according to all substrate types involved in a protocol (F, N, S, Gp), independent of the sequence of titrations of substrates and inhibitors which define the Electron-transfer-pathway states. The N-type substrates are listed in parentheses, independent of the sequence of titrations. ROX states may or may not be included in a SUIT protocol, which does not change its category. Similarly, the CIV assay may or may not be added at the end of a SUIT protocol, without effect on the category of a SUIT protocol.
|
| Cell count and normalization in HRR | Nce | The cell count Nce is the number of cells, expressed in the abstract unit [x] (1 Mx = 106 x). The elementary entity cell Uce [x] is the real unit, the 'single individual cell'. A cell count is the multitude or number N of cells, Nce = N·Uce (Gnaiger MitoFit Preprints 2020.4). Normalization of respiratory rate by cell count yields oxygen flow IO2 expressed in units [amol·s-1·x-1] (=10-18 mol·s-1·x-1). |
| Cellular substrates | Ce; Cm | (1) Cellular substrates in vivo, endogenous; Ce.
(2) Cellular substrates in vivo, with exogenous substrate supply from culture medium or serum; Cm.
|
| Channel | F7 | » See O2k signals and output |
| Charge | Qel [C] | Charge Qel is the quantity of electricity expressed in the SI unit coulomb [C]. QelX [C] indicates the charge carried by the quantity of a specified ion X. |
| Charge number | zX | The charge number of an ion X or electrochemical reaction with unit stoichiometric number of X is the particle charge [C·x-1] divided by the elementary charge [C·x-1]. The particle charge QNX is the charge per count of ions X or per ion X transferred in the reaction as defined in the reaction equation. |
| Chemical background | CHB, Chb |  Chemical background Chb is due to autooxidation of the reagents. During CIV assays, ascorbate and TMPD are added to maintain cytochrome c in a reduced state. External cytochrome c may be included in the CIV assay. The autooxidation of these compounds is linearly oxygen-dependent down to approximately 50 µM oxygen and responsible for the chemical background oxygen flux after the inhibition of CIV. Oxygen flux due to the chemical reaction of autooxidation must be corrected for the instrumental O2 background. The correction for chemical background is necessary to determine CIV activity, in which case the instrumental O2 background and chemical background may be combined in an overall correction term. Chemical background Chb is due to autooxidation of the reagents. During CIV assays, ascorbate and TMPD are added to maintain cytochrome c in a reduced state. External cytochrome c may be included in the CIV assay. The autooxidation of these compounds is linearly oxygen-dependent down to approximately 50 µM oxygen and responsible for the chemical background oxygen flux after the inhibition of CIV. Oxygen flux due to the chemical reaction of autooxidation must be corrected for the instrumental O2 background. The correction for chemical background is necessary to determine CIV activity, in which case the instrumental O2 background and chemical background may be combined in an overall correction term. |
| Chemical potential | µB [J/mol] | The chemical potential of a substance B, µB [J/mol], is the partial derivative of Gibbs energy, G [J], per amount of B, nB [mol], at constant temperature, pressure, and composition other than that of B,
µB = (∂G/∂nB)T,p,nj≠B The chemical potential of a solute in solution is the sum of the standard chemical potential under defined standard conditions and a concentration (activity)-dependent term, µB = µB° + RT ln(aB)The standard state for the solute is refered to ideal behaviour at standard concentration, c° = 1 mol/L, exhibiting infinitely diluted solution behaviour [1]. µB° equals the standard molar Gibbs energy of formation, ΔfGB° [kJ·mol-1]. The formation process of B is the transformation of the pure constituent elements to one mole of substance B, with all substances in their standard state (the most stable form of the element at 100 kPa (1 bar) at the specified temperature) [2]. |
| Chinese Society of Mitochondrial Research and Medicine | Chinese-Mit | The Chinese Society of Mitochondrial Research and Medicine (Chinese-Mit) is a member of ASMRM. |
| Chloroplasts | pt? | Chloroplasts (Greek chloros: green; plastes: the one who forms) are small structures within the cells that conduct photosynthesis. They are a type of organelle called plastids that are present in eukaryotic plant cells (algae, aquatic and terrestrial plants) and characterized by having two membranes and a high concentration of the pigment Chlorophyll. Like mitochondria, they originated through the endosymbiosis of a cyanobacteria by an early eukaryotic cell and they have their own DNA which replicates during cell division. In addition to photosynthesis, in their internal matrix called stroma they also carry out other metabolic functions within the plant cells such as fatty acid synthesis or amino acid synthesis. |
| Citrate synthase | CS | Condensation of oxaloacetate with acetyl-CoA yields citrate as an entry into the TCA cycle. CS is located in the mt-matrix. CS activity is frequently used as a functional marker of the amount of mitochondria (mitochondrial elementary marker, mtE) for normalization of respiratory flux. |
| Closed chamber | C | The O2k-chamber can be used as a closed system or open system. Gas bubbles must be avoided. |
| Coenzyme A | CoA | Coenzyme A is a coenzyme playing an essential role in the tricarboxylic acid cycle (oxidation of pyruvate to acetyl-CoA) and fatty acid oxidation. CoA is a thiol that reacts with carboxylic acids to form CoA-activated thioesters. |
| Coenzyme Q | Q, CoQ | Coenzyme Q or ubiquinone (2,3-dimethoxy-5-methyl-6-polyprenyl-1,4-benzoquinone) was discovered in 1957 by the group of Crane. It is a lipid composed of a benzoquinone ring with an isoprenoid side chain, two methoxy groups and one methyl group. The length of the isoprenoid chain varies depending on the species; for example, six isoprenoid units (CoQ6) is the most commonly found CoQ in Saccharomyces cerevisiae, eight units in Escherichia coli (CoQ8), nine units in Caenorhabditis elegans and rodents (CoQ9), ten units in humans (CoQ10), and some species have more than one CoQ form, e.g. human and rodent mitochondria contain different proportions of CoQ9 and CoQ10. These redox compounds exist in three different forms: quinone (oxidized), quinol (reduced), and an intermediate semiquinone. More details » Q-junction |
| Coenzyme Q2 | CoQ2 | Coenzyme Q2 or ubiquinone-2 (CoQ2) is a quinone derivate composed of a benzoquinone ring with an isoprenoid side chain consisting of two isoprenoid groups, with two methoxy groups, and with one methyl group. In HRR it is used as a Q-mimetic to detect the redox changes of coenzyme Q at the Q-junction in conjunction with the Q-Module, since the naturally occurring long-chain coenzyme Q (e.g. CoQ10) is trapped within membrane boundaries. CoQ2 can react both with mitochondrial complexes (e.g. CI, CII and CIII) at their quinone-binding sites and with the detecting electrode. |
| Comma for separating a term and its abbreviation | , | Should we used a comma for separating a term and its abbreviation in the text? The SI Brochure frequently does not use a comma. The comma might be added, if it helps to clarify the distinction between the term and its abbreviation. The example “reduced Q fraction, Qr” – the sequence of Q and Qr may be confusing without comma. There will always be examples, where it is not clear, if a comma is needed. |
| Company of Scientists | CompaSci | The Company of Scientists evolves as a concept for implementing scientific innovations on the market. |
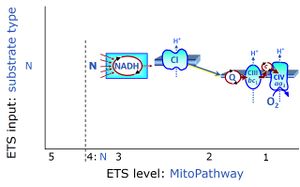
| Complex I | CI | Complex I, NADH:ubiquinone oxidoreductase (EC 1.6.5.3), is an enzyme complex of the Electron transfer pathway, a proton pump across the inner mt-membrane, responsible for electron transfer to ubiquinone from NADH formed in the mt-matrix. CI forms a supercomplex with Complex III. There is a widespread ambiguity on the 'lonely H+ (the lonely hydron)' surrounding Complex I: CI ambiguities. |
| Complex I&II-linked substrate state | NS | See NS-pathway control state (previous: CI&II-linked) |
| Complex I-linked substrate state | N | See N-pathway control state (previous: CI-linked) versus Complex I |
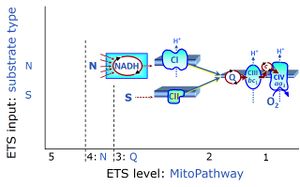
| Complex II | CII | Complex II or succinate:quinone oxidoreductase (SQR) is the only membrane-bound enzyme in the TCA cycle and is part of the electron transfer pathway. The reversible oxidoreduction of succinate and fumarate is catalyzed in a soluble domain and coupled to the reversible oxidoreduction of quinol and quinone in the mitochondrial inner membrane. CII consists in most species of four subunits. The flavoprotein succinate dehydrogenase is the largest polypeptide of CII, located on the matrix face of the mt-inner membrane. Succinate:quinone oxidoreductases (SQRs, SDHABCD) favour oxidation of succinate and reduction of quinone in the canonical forward direction of the TCA cycle and electron transfer into the Q-junction. In contrast, quinol:fumarate reductases (QFRs, fumarate reductases, FRDABCD) tend to operate in the reverse direction reducing fumarate and oxidizing quinol. |
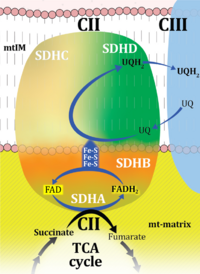
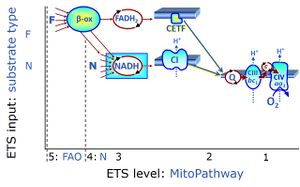
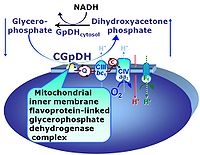
| Complex II ambiguities | CII ambiguities | The current narrative that the reduced coenzymes NADH and FADH2 feed electrons from the tricarboxylic acid (TCA) cycle into the mitochondrial electron transfer system can create ambiguities around respiratory Complex CII. Succinate dehydrogenase or CII reduces FAD to FADH2 in the canonical forward TCA cycle. However, some graphical representations of the membrane-bound electron transfer system (ETS) depict CII as the site of oxidation of FADH2. This leads to the false believe that FADH2 generated by electron transferring flavoprotein (CETF) in fatty acid oxidation and mitochondrial glycerophosphate dehydrogenase (CGpDH) feeds electrons into the ETS through CII. In reality, NADH and succinate produced in the TCA cycle are the substrates of Complexes CI and CII, respectively, and the reduced flavin groups FMNH2 and FADH2 are downstream products of CI and CII, respectively, carrying electrons from CI and CII into the Q-junction. Similarly, CETF and CGpDH feed electrons into the Q-junction but not through CII. The ambiguities surrounding Complex II in the literature call for quality control, to secure scientific standards in current communications on bioenergetics and support adequate clinical applications. |
| Complex II-linked substrate state | SRot, S | See S-pathway control state (previous: CII-linked) |
| Complex III | CIII | Complex III or coenzyme Q : cytochrome c - oxidoreductase, sometimes also called the cytochrome bc1 complex is a complex of the electron transfer pathway. It catalyzes the reduction of cytochrome c by oxidation of coenzyme Q (CoQ) and the concomitant pumping of 4 protons from the cathodic (negative) mitochondrial matrix to the anodic (positive) intermembrane space. |
| Complex IV | CIV | Complex IV or cytochrome c oxidase is the terminal oxidase of the mitochondrial electron transfer system, reducing oxygen to water, with reduced cytochrome c as a substrate. Concomitantly to that, CIV pumps protons against the electrochemical protonmotive force. CIV is frequently abbreviated as COX or CcO. It is the 'ferment' (Atmungsferment) of Otto Warburg, shown to be related to the cytochromes discovered by David Keilin. |
| Concentration | c [mol·L-1]; C [x·L-1] | Concentration [mol·L-1] is a volume-specific quantity for diluted samples s. In a concentration, the sample is expressed in a variety of formats: count, amount, charge, mass, energy. In solution chemistry, amount concentration is amount of substance nB per volume V of the solution, cB = [B] = nB·V-1 [mol·dm-3] = [mol·L-1]. The standard concentration, c°, is defined as 1 mol·L-1 = 1 M. Count concentration CX = NX·V-1 [x·L-1] is the concentration of the number NX of elementary entities X, for which the less appropriate term 'number concentration' is used by IUPAC. If the sample is expressed as volume Vs (e.g., VO2), then the 'volume-concentration' of Vs in V is termed 'volume fraction', Φs = Vs·V-1 (e.g., volume fraction of O2 in dry air, ΦO2) = 0.20946). Density is the mass concentration in a volume VS of pure sample S. A change of concentration, dcX, in isolated or closed systems at constant volume is due to internal transformations (advancement per volume) only. In closed compressible systems (with a gas phase), the concentration of the gas changes, when pressure-volume work is performed on the system. In open systems, a change of concentration can additionally be due to external flow across the system boundaries. |
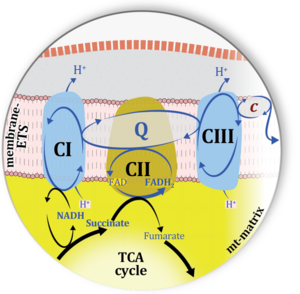
| Convergent electron flow | n.a. | Convergent electron flow is built into the metabolic design of the Electron transfer pathway. The glycolytic pathways are characterized by important divergent branchpoints: phosphoenolpyruvate (PEPCK) branchpoint to pyruvate or oxaloactetate; pyruvate branchpoint to (aerobic) acetyl-CoA or (anaerobic) lactate or alanine. The mitochondrial Electron transfer pathway, in contrast, is characterized by convergent junctions: (1) the N-junction and F-junction in the mitochondrial matrix at ET-pathway level 4, with dehydrogenases (including the TCA cycle) and ß-oxidation generating NADH and FADH2 as substrates for Complex I and electron-transferring flavoprotein complex, respectively, and (2) the Q-junction with inner mt-membrane respiratory complexes at ET-pathway level 3, reducing the oxidized ubiquinone and partially reduced semiquinone to the fully reduced ubiquinol, feeding electrons into Complex III. |
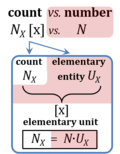
| Count | NX [x] | Count NX is the number N of elementary entities of entity-type X. The single elementary entity UX is a countable object or event. NX is the number of objects of type X, whereas the term 'entity' and symbol X are frequently used and understood in dual-message code indicating both (1) the entity-type X and (2) a count of NX = 1 x for a single elementary entity UX. 'Count' is synonymous with 'number of entities' (number of particles such as molecules, or objects such as cells). Count is one of the most fundamental quantities in all areas of physics to biology, sociology, economy and philosophy, including all perspectives of the statics of countable objects to the dynamics of countable events. The term 'number of entities' can be used in short for 'number of elementary entities', since only elementary entities can be counted, and as long as it is clear from the context, that it is not the number of different entity types that are the object of the count. |
| Coupling-control protocol | CCP | A coupling-control protocol CCP induces different coupling control states at a constant electron-transfer-pathway state. Residual oxygen consumption (Rox) is finally evaluated for Rox correction of flux. The CCP may be extended, when further respiratory states (e.g. cell viability test; CIV assay) are added to the coupling control module consisting of three coupling control states. The term phosphorylation control protocol, PCP, has been introduced synonymous for CCP. » MiPNet article |
| Coupling-control ratio | CCR | Coupling-control ratios CCR are flux control ratios FCR at a constant mitochondrial pathway-control state. In mitochondrial preparations, there are three well-defined coupling states of respiration: LEAK respiration, OXPHOS, and Electron-transfer-pathway state (ET state). In these states, the corresponding respirtory rates are symbolized as L, P, and E. In living cells, the OXPHOS state cannot be induced, but in the ROUTINE state the respiration rate is R. A reference rate Z is defined by taking Z as the maximum flux, i.e. flux E in the ET-state, such that the lower and upper limits of the CCR are defined as 0.0 and 1.0. Then there are two mitochondrial CCR, L/E and P/E, and two CCR for living cells, L/E and R/E. |
| Coupling-control state | CCS | Coupling-control states are defined in mitochondrial preparations (isolated mitochondria, permeabilized cells, permeabilized tissues, homogenates) as LEAK respiration, OXPHOS, and ET states, with corresponding respiration rates (L, P, E) in any electron-transfer-pathway state which is competent for electron transfer. These coupling states are induced by titration of ADP and uncouplers, and application of specific inhibitors of the phosphorylation pathway. In living cells, the coupling-control states are LEAK respiration, ROUTINE, and ET states of respiration with corresponding rates L, R, E, using membrane-permeable inhibitors of the phosphorylation system (e.g. oligomycin) and uncouplers (e.g. CCCP). Coupling-control protocols induce these coupling-control states sequentially at a constant electron-transfer-pathway state. |
| Coupling/pathway control diagram | CPCD | Coupling/pathway control diagrams illustrate the respiratory states obtained step-by-step in substrate-uncoupler-inhibitor titrations in a SUIT protocol. Each step (to the next state) is defined by an initial state and a metabolic control variable, X. The respiratory states are shown by boxes. X is usually the titrated substance in a SUIT protocol. If X (ADP, uncouplers, or inhibitors of the phosphorylation system, e.g. oligomycin) exerts coupling control, then a transition is induced between two coupling-control states. If X (fuel substrates, e.g. pyruvate and succinate, or Electron transfer pathway inhibitors, e.g. rotenone) exerts pathway control, then a transition is induced between two Electron-transfer-pathway states. The type of metabolic control (X) is shown by arrows linking two respiratory states, with vertical arrows indicating coupling control, and horizontal arrows indicating pathway control. Marks define the section of an experimental trace in a given respiratory state (steady state). Events define the titration of X inducing a transition in the SUIT protocol. The specific sequence of coupling control and pathway control steps defines the SUIT protocol pattern. The coupling/pathway control diagrams define the categories of SUIT protocols (see Figure). |
| Creatine | Cr | Creatine is a nitrogenous organic acid that occurs naturally in vertebrates and helps primarily muscle cells to supply energy by increasing the formation of adenosine triphosphate (ATP). |
| Creatine kinase | CK | The mitochondrial creatine kinase, also known as phosphocreatine kinase (CPK), facilitates energy transport with creatine and phosphocreatine as diffusible intermediates. |
| Critical oxygen pressure | pc | The critical oxygen pressure, pc, is defined as the partial oxygen pressure, pO2, below which aerobic catabolism (respiration or oxygen consumption) declines significantly. If anaerobic catabolism is activated simultaneously to compensate for lower aerobic ATP generation, then the limiting oxygen pressure, pl, is equal to the pc. In many cases, however, the pl is substantially lower than the pc. |
| Cross-linked respiratory states | CLRS | Coordinated respiratory SUIT protocols are designed to include cross-linked respiratory states, which are common to these protocols. Different SUIT protocols address a variety of respiratory control steps which cannot be accomodated in a single protocol. Cross-linked respiratory states are included in each individual coordinated protocol, such that these states can be considered as replicate measurements, which also allow for harmonization of data obtained with these different protocols. |
| Cyanide | KCN | Cyanide (usually added as KCN) is a competitive inhibitor of cytochcrome c oxidase (CIV). Inhibition is reversed by pyruvate and high oxygen levels. |
| Cyclic voltammetry | CV | Cyclic voltammetry (CV) is a type of electrochemical measurement which is applied with the Q-Module as quality control to
(1) determine the oxidation and reduction peak potentials of Coenzyme Q in the specific experimental condition, (2) check the quality of the Q-Sensor, and (3) test the interference of chemicals used in the HRR assay with the Q-Sensor. In CV, the Q-Sensor with the three-electrode system is used to obtain information about the analyte (CoQ) by measuring the current (I) as the electric potential (V) between two of the electrodes is varied. In CV the electric potential between the glassy carbon (GC) and the Ag/AgCl reference electrode changes linearly versus time in cyclical phases, while the current is detected between GC and platinum electrode (Pt). The detected current is plotted versus the applied voltage to obtain the typical cyclic voltammogram trace (Figure 1). The presence of substances that are oxidized/reduced will result in current between GC and Pt, which can be seen as characteristic peaks in the voltammogram at a defined potential. The oxidation or the reduction peak potential values are used to set the GC (integrated into the Q-Sensor) for a separate experiment to measure the Q redox state of a biological sample. The oxidation and reduction peak potentials can be influenced by 1) the respiration medium, 2) the type of CoQ, 3) the polarization window, 4) the scan speed, 5) the number of cycles, 6) the concentration of the analyte (CoQ), and 7) the initial polarization voltage. <be> |
| Cyclosporin A | CsA | Cyclosporin A (CsA) is a cyclic undecapeptide from an extract of soil fungi that binds the cyclophilin D and thus preventing the formation of the mitochondrial permeability transition pore. The interaction of CsA with the cyclophilin D is phosphate mediated but the full mechanism of interaction is not well understood. For example, the deficiency of cyclophilin D in KO models does not prevent mitochondria from permeability transition and from CsA inhibition. Moreover, it is also a is a calcineurin inhibitor and potent immunosuppressive agent used largely as a means of prophylaxis against cellular rejection after solid organ transplantation. |
| Cytochrome c | c | Cytochrome c is a component of the Electron transfer-pathway (Electron transfer pathway) in mitochondria. It is a small heme protein loosely associated with the outer side of the inner mitochondrial membrane. The heme group of cytochrome c transfers electrons from Complex III to Complex IV. The release of cytochrome c into the cytoplasm is associated with apoptosis. Cytochrome c is applied in HRR to test the integrity of the mitochondrial outer membrane (cytochrome c control efficiency). |
| Cytochrome c control efficiency | jcyt c | The cytochrome c control efficiency expresses the control of respiration by externally added cytochrome c, c, as a fractional change of flux from substrate state CHNO to CHNOc. These fluxes are corrected for Rox and may be measured in the OXPHOS state or ET state, but not in the LEAK state. In this flux control efficiency, CHNOc is the reference state with stimulated flux; CHNO is the background state with CHNO substrates, upon which c is added: jcyt c = (JCHNOc-JCHNO)/JCHNOc. |
| D-number | D### | D number is the unique code given for each SUIT protocol. In the same SUIT protocol family (SUIT-###), there might be different protocols, specifically designed for different sample type (e.g., different mitochondrial preparations) or for different applications (e.g., O2, AmR, Fluo, MgG). Since the use of different kinds of sample or application may result in slightly different steps, each protocol receives a different D-number. |
| DORA | DORA | The Declaration on Research Assessment DORA recognizes the need to improve the ways in which researchers and the outputs of scholarly research are evaluated. |
| DTPA | DTPA | DTPA (Diethylenetriamine-N,N,N',N,N-pentaacetic acid, pentetic acid,(Carboxymethyl)imino]bis(ethylenenitrilo)-tetra-acetic acid) is a polyaminopolycarboxylic acid (like EDTA) chelator of metal cations. DTPA wraps around a metal ion by forming up to eight bounds, because each COO- group and and N-center serves a center for chelation. With transition metals the number of bounds is less than eight. The compound is not cell membrane permeable. In general, it chelates multivalent ions stronger than EDTA. |
| DatLab 2 | DL2 | DatLab 2 (DL2) is a MS-DOS programe. DL2 is still used for analysis of oxygen kinetics, after exporting files recorded in recent DatLab versions. A user-friendly O2-kinetics module is in preparation (DL8). |
| DatLab data file | dld8, DLD | DatLab 8: The file type generated is *.dld8. DatLab 7: The file type generated is *.DLD. |
| Data recording interval | F7 | The data recording interval is the time interval at which data is sampled with an instrument. In DatLab the data recording interval is set in the O2k control window. The system default value is 2 s. A lower data recording interval is selected for kinetic experiments, and when the volume-specific oxygen flux is high (>300 pmol O2·s-1·ml-1). Technically, the O2k instrument (hardware) measures the sensor signal every 10ms (which is NOT the „data recording interval“). By the given data recording interval from DatLab (software) a discrete number of sensor signal points are taken to calculate an average value in the O2k (e.g. a data recording interval of 2 s can take 200 sensor signal points; a data recording interval of 0.5 s can take 50 sensor signal points). This average value is sent to DatLab and is recorded as a raw data point. However, there is a defined threshold: the O2k does not apply more than 200 sensor signal points to calculate the average for the raw data point. For example a data recording interval of 3 s could take 300 sensor signal points but only the 200 most recent sensor signal points are used for averaging. |
| DataCite | DataCite | DataCite is a global community of organizations and researchers identifying and citing research outputs and resources. We provide services to create persistent records of research, enable discovery and reuse, and support workflows throughout the research lifecycle. |
| Dead cells | dce | Dead cells dce are characterized by the loss of plasma membrane barrier function. The total cell count (Nce) is the sum of viable cells (Nvce) and dead cells (Ndce). |
| Decimal marker and spaces between groups of numerals | . | A decimal marker is used to separate the integral part of numbers from the decimal part. The SI recommends: "the symbol for the decimal marker shall be either the point on the line or the comma on the line". In English language versions, the dot (point on the line) should be used uniquely as the decimal marker. To avoid ambiguities, BEC follows the SI recommendation that “Numbers may be divided in groups of three in order to facilitate reading; neither dots nor commas are ever inserted in the spaces between groups” (pages 183-184). |
| Density | ρ, C, D | Density, mass density ρ = m·V-1 [kg·m-3], is mass m divided by volume V. Surface density ρA = m·A-1 [kg·m-2] (SI). For a pure sample S, the mass density ρS = mS·VS-1 [kg·m-3] is the mass m of pure sample S per volume VS of the pure sample. With density ρ thus defined, the 'amount density' of substance B is ρB = nB·VB-1 [mol·m-3]. This is not a commonly used expression, but the inverse is defined as the molar volume of a pure substance (IUPAC), Vm,B = VB·nB-1 [m3·mol-1]. The pure sample is a pure gas, pure liquid or pure solid of a defined elementary entity. The amount concentration, cB = nB·V-1 [mol·m-3] is the amount nB of substance B divided by the volume V of the mixture (IUPAC), and this is not called an 'amount density'. The term 'amount density' is reserved for an amount of substance per volume VS of the pure substance. This explicit distinction between 'density' related to the volume of the sample and 'concentration' related to the total volume of the mixture is very helpful to avoid confusion. Further clarification is required in cases, when the mass density ρs of the sample in the mixture differs from the mass density ρS of the pure sample before mixing. Think of a sample S of pure ethanol with a volume of 1 L at 25 °C, which is mixed with a volume of 1 L of pure water at 25 °C: after the temperature of the mixture has equilibrated to 25 °C, the total volume of the mixture is less than 2 L, such that the volume VS of 1 L pure ethanol has diminished to a smaller volume Vs of ethanol in the mixture; the density of ethanol in the mixture is higher than the density of pure ethanol (this is incomplete additivity). The volume Vs of sample s in a mixture is by definition smaller than the total volume V of the mixture. Sample volume VS and system volume V are identical, but this applies only to the case of a pure sample. Concentration is related to samples s per total volume V of the mixture, whereas density is related to samples S or s per volume VS = V or Vs < V, respectively (BEC 2020.1). |
| Deselect channels | F7 | Channels can be selected/deselected in DatLab in the O2k configuration. Deselect all O2k-MultiSensor channels in O2k-Core applications. Select only the specifically used channels in O2k-MultiSensor applications. |
| Dicarboxylate carrier | DIC | The dicarboxylate carrier is a transporter which catalyses the electroneutral exchange of malate2- (or succinate2-) for inorganic phosphate, HPO42-. |
| Digital Object Identifier | DOI | A Digital Object Identifier, DOI, is a persistent identifier used to uniquely identify online publications in order to ensure they remain traceable, searchable and citable over the long term. Compared to other types of persistent identifiers, the DOI system is widespread and well established in the life sciences arena, and it provides widely accepted visible proof that a publication is citable. |
| Digitonin | Dig | Digitonin is a mild detergent that permeabilizes plasma membranes selectively due to their high cholesterol content, whereas mt-membranes with lower cholesterol content are affected only at higher concentrations. Digitonin is a natural product and thus the effective concentration has to be determined by titrations for every batch. The optimum effective digitonin concentrations for complete plasma membrane permeabilization of cultured cells can be determined directly in a respirometric protocol (see: SUIT-010 O2 ce-pce D008). |
| Dihydro-orotate dehydrogenase | DhoDH | Dihydro-orotate dehydrogenase is an electron transfer complex of the inner mitochondrial membrane, converting dihydro-orotate (Dho) into orotate, and linking electron transfer through the Q-junction to pyrimidine synthesis and thus to the control of biogenesis. |
| Dihydroethidium | DHE | Dihydroethidium (also called hydroethidine) is a cell permeant fluorescent probe used to analyse superoxide presence. It is a reduced form of ethidium that presents blue fluorescence, and after oxidation by superoxide becomes able to intercalate DNA and emits red fluorescence (excitation wavelength ~518–535 nm, emission ~605–610 nm). It has been used to detect superoxide by HPLC and by fluorescence microscopy. |
| Dimethyl sulfoxide | DMSO | Dimethyl sulfoxide is a polar aprotic solvent that dissolves both polar and nonpolar compounds and is miscible in a wide range of organic solvents as well as water. DMSO may also be used as a cryoprotectant, added to cell media to reduce ice formation and thereby prevent cell death during the freezing process. |
| Dinitrochlorobenzene | DNCB | Dinitrochlorobenzene (1-chloro-2,4-dinitrobenzene) (DNCB) is a glutathione (GSH) inhibitor. |
| Dinitrophenole | DNP | 2,4-dinitrophenole (C6H4N2O5; M = 184.11 g·mol-1) is a protonophore acting as an uncoupler of oxidative phosphorylation. |
| Directory of Open Access Journals | DOAJ | The Directory of Open Access Journals is a free online directory that indexes and provides access to open access peer-reviewed journals. |
| Dithionite | Dit Dit - (The abbreviation 'Dith' has been used previously and is stepwise replaced by Dit.) | The sodium salt of Dithionite Na2S2O4 (Dit) is the 'zero oxygen solution powder' used for calibration of oxygen sensors at zero oxygen concentration, or for stepwise reduction of oxygen concentrations in instrumental O2 background tests. It is not recommended to use dithionite in experiments with biological samples or several multisensor approaches, for these see Setting the oxygen concentration. |
| Duroquinol | DQ | ET-pathway level 2 is supported by duroquinol DQ feeding electrons into Complex III (CIII) with further electron transfer to CIV and oxygen. Upstream pathways are inhibited by rotenone and malonic acid in the absence of other substrates linked to ET-pathways with entry into the Q-junction. |
| E | e, E | » Energy, Exergy E
» elementary charge e = 1.602 176 634∙10-19 C∙x-1 » Euler's number e ~ 2.718 281 828 459 » ET capacity E |
| E-L coupling efficiency | jE-L | |
| E-L net ET capacity | E-L | |
| E-P control efficiency | jE-P | |
| E-P excess capacity | E-P | |
| E-R control efficiency | jE-R | |
| E-R reserve capacity | E-R | |
| ET capacity | E | |
| ET-pathway substrate types | n.a. | See Electron-transfer-pathway state |
| Elamipretide | Bendavia | Bendavia (Elamipretide) was developed as a mitochondria-targeted drug against degenerative diseases, including cardiac ischemia-reperfusion injury. Clinical trials showed variable results. It is a cationic tetrapeptide which readily passes cell membranes, associates with cardiolipin in the mitochondrial inner membrane. Supercomplex-associated CIV activity significantly improved in response to elamipretide treatment in the failing human heart. |
| Elasticity | ε | According to David Fell, "Elasticities are properties of individual enzymes and not the metabolic system. The elasticity of an enzyme to a metabolite is related to the slope of the curve of the enzyme's rate plotted against metabolite concentration, taken at the metabolite concentrations found in the pathway in the metabolic state of interest. It can be obtained directly as the slope of the logarithm of the rate plotted against the logarithm of the metabolic concentration. The elasticity will change at each point of the curve (s,v) and must be calculated for the specific concentration of the metabolite (s) that will give a specific rate (r) of the enzyme activity" (See Figure).
 |
| Electric current | Iel [A = C·s-1]; [mol·s-1]; [x·s-1] | Current or electric flow Iel is the advancement of charge per unit of time, expressed in the SI base unit ampere [C·s-1 = A]. Electrons or ions are the current-carrying motive entities of electric flow. Electrons e- are negatively charged subatomic particles carrying 'negative electricity' with a mass that is about 1/1700 of the smallest particle — the proton — carrying 'positive electricity' (Thompson 1906). Correspondingly the velocity of electrons is much higher than that of protons or any other (larger) ion. Current is the velocity v of paticles times the number of motive charges. Therefore, electron current Ie- is of a different nature from electric current Ielχ carried by all species i of ions Xi (cations and anions) summarized as χ = Σ(zi·Xi). Whereas Ie- is the net translocation of electrons moving forwards and backwards, Ielχ is the net translocation of charges carried by different cations and anions. In contrast, ion current IelX of a specific ion X is the partial translocation of charges carried by net translocation of ion X only. If cation current IelX+ is antagonized entirely by counterion current IelY- as the process of antiport, then the electric current Ielχ is zero. The (net) electric current in a compartmental system is driven by the electric force ΔelFp+ or electric potential difference ΔΨp+, whereas a compensated ion/counterion antiport current is insensitive to the electric potential difference. |
| Electric current density | j [C·m-2] | Electric current density is current divided by area, j=I·A-1 [C·m-2]. Compare: density. |
| Electrochemical constant | f [J·C-1·K-1] | The electrochemical constant f has the SI unit for energy per charge per temperature [J·C-1·K-1].
f = k·e-1, the Boltzmann constant k divided by the elementary charge e. f = R·F-1, the gas constant R divided by the Faraday constant F. |
| Electron flow | Ie | Electron flow through the mitochondrial Electron transfer pathway (ET-pahway) is the scalar component of chemical reactions in oxidative phosphorylation (OXPHOS). Electron flow is most conveniently measured as oxygen consumption (oxygraphic measurement of oxygen flow), with four electrons being taken up when oxygen (O2) is reduced to water. |
| Electron transfer pathway | ET pathway | In the mitochondrial electron transfer pathway (ET pathway) electrons are transferred from externally supplied reduced fuel substrates to oxygen. Based on this experimentally oriented definition (see ET capacity), the ET pathway consists of (1) the membrane-bound ET pathway with respiratory complexes located in the inner mt-membrane, (2) TCA cycle and other mt-matrix dehydrogenases generating NADH and succinate, and (3) the carriers involved in metabolite transport across the mt-membranes. » MiPNet article |
| Electron-transfer-pathway state | ET-pathway state |
Electron-transfer-pathway states are obtained in mitochondrial preparations (isolated mitochondria, permeabilized cells, permeabilized tissues, tissue homogenate) by depletion of endogenous substrates and addition to the mitochondrial respiration medium of fuel substrates (CHNO) activating specific mitochondrial pathways, and possibly inhibitors of specific pathways. Mitochondrial electron-transfer-pathway states have to be defined complementary to mitochondrial coupling-control states. Coupling-control states require ET-pathway competent states, including oxygen supply. Categories of SUIT protocols are defined according to mitochondrial ET-pathway states. » MiPNet article |
| Electron-transferring flavoprotein Complex | CETF | Electron-transferring flavoprotein Complex (CETF) is a respiratory Complex localized at the matrix face of the inner mitochondrial membrane, supplies electrons to Q, and is thus an enzyme Complex of the mitochondrial Electron transfer pathway (ET-pathway). CETF links the ß-oxidation cycle with the membrane-bound electron transfer system in fatty acid oxidation (FAO). |
| Elementary charge | e [C·x-1] | The elementary charge or proton charge e has the SI unit coulomb [C], but more strictly coulomb per elementary unit [C·x-1]. -e is the charge per electron. Elementary charge e is the charge per elementary entity H+ with SI unit [C] but canonical SI unit [C·x-1]. When the charge Qel [C] of a number Ne [x] of electrons e is divided by the count Ne, then the particle charge QNX (QNX) charge per elementary entity is obtained, -e = Qel/Ne [C·x-1]. e is also used as an atomic unit. |
| Elementary entity | UX [x] |
An elementary entity is an entity of type X, distinguished as a single unit of countable objects (X = molecules, cells, organisms, particles, parties, items) or events (X = beats, collisions, emissions, decays, celestial cycles, instances, occurrences, parties). "An elementary entity may be an atom, a molecule, an ion, an electron, any other particle or specified group of particles" (Bureau International des Poids et Mesures 2019). An elementary entity, therefore, needs to be distinguished from non-countable entities and the general class of entities X. This distinction is emphasized by the term 'elementary' (synonymous with 'elementary entity') with symbol UX and elementary unit [x]. If an object is defined as an assembly of particles (a party of two, a molecule as the assembly of a stoichiometric number of atoms), then the elementary is the assembly but not the assembled particle. A number of defined elementaries UX is a count, NX = N·UX [x], where N is a number, and as such N is dimensionless, and N is a number (stop) and is not 'a number of ..'. Elementaries are added as items to a count. The elementary UX has the dimension U of the count NX. The elementary UX has the same unit [x] as the count NX, or more accurately it gives the count the defining 'counting-unit', which is the 'elementary unit' [x]. From the definition of count as the number (N) of elementaries (U) of entity type X, it follows that count divided by elementary is a pure number, N = NX·UX-1. The unit x of a count can neither be the entity X nor a number. The elementary of type X defines the identity X of the elementary UX with the unit 'elementary unit' with symbol [x]. Since a count NX is the number of elementary entities, the elementary UX is not a count (UX is not identical with N·UX). |
| Elementary unit | x | The elementary unit [x] is the unit of a count NX [x]. The International System of Units defines the unit of a count as 1. Then the Number 1 is the Unit of the Count of Entities — NUCE. This causes a number of formal inconsistencies which are resolved by introducing the elementary unit [x] as the abstracted unit of Euclid’s unit, which is an elementary entity UX [x], and as the unit of Euclid’s number, which is a count NX [x]. |
| Energy | E; various [J] | Heat and work are forms of energy [1 cal = 4.184 J]. Energy [J] is a fundamental term that is used in physics and physical chemistry with various meanings [1]. These meanings become explicit in the following equations relating to systems at constant volume (dV = 0) or constant gas pressure (dp = 0). Energy is exchanged between a system and the environment across the system boundaries in the form of heat, deQ, total or available work, detW (or detW), and matter, dmatU (or dmatH) [2],
dU = (deQ + detW) + dmatU ; dV = 0 [Eq. 1a] dH = (deQ + deW) + dmatH ; dp = 0 [Eq. 1b] Whereas dU (or dH) describe the internal-energy change (or enthalpy change) of the system, heat and work are external energy changes (subscript e; et: external total; e: external excluding pressure-volume work), and dmatU (or dmatH) are the exchange of matter expressed in internal-energy (or enthaply) equivalents. In closed systems, dmatU = 0 (dmatH = 0). The energy balance equation [Eq. 1] is a form of the First Law of Thermodynamics, which is the law of conservation of internal-energy, stating that energy cannot be generated or destroyed: energy can only be transformed into different forms of work and heat, and transferred in the form of matter. Notably, the term energy is general and vague, since energy may be associated with either the first or second law of thermodynamics. Work is a form of energy exchange [Eq. 1], but can be seen as exergy exchange in conjunction with deG = deW in a closed system [Eq. 3b]. An equally famous energy balance equation considers energy changes of the system only, in the most simple form for isothermal systems (dT = 0): dU = dA + T∙dS = dU + dB [Eq. 2a] dH = dG + T∙dS = dG + dB [Eq. 2b] The internal-energy change, dU (enthalpy change, dH) is the sum of free energy change (Helmholtz energy, dA; or Gibbs energy = exergy change, dG) and bound energy change (bound energy, dB = T∙dS). The bound energy is that part of the energy change that is always bound to an exchange of heat. A third energy balance equation accounts for changes of the system in terms of irreversible internal processes (i) occuring within the system boundaries, and reversible external processes (e) of transfer across the system boundaries (at constant gas pressure), dH = diH + deH [Eq. 3a] dG = diG + deG [Eq. 3b] The energy conservation law of thermodynamics (first law) can be formulated as diH = 0 (at constant gas pressure), whereas the generally negative sign of the dissipated energy, diG ≡ diD ≤ 0, is a formulation of the second law of thermodynamics. Insertion into Eq. 3 yields, dH = deH [Eq. 4a] dG = diD + deW + dmatG [Eq. 4b]When talking about energy transformations, the term energy is used in a general sense without specification of these various forms of energy. |
| Energy charge | AEC | The energy charge of the adenylate system or adenylate energy charge (AEC) has been defined by Atkinson and Walton (1967) as (ATP + ½ ADP)/(AMP + ADP + ATP). Wheather the AEC is a fundamental metabolic control parameter remains a controversial topic. |
| Enthalpy | H [J] | Enthalpy, H [J], can under conditions of constant gas pressure neither be destroyed nor created (first law of thermodynamics: diH/dt = 0). The distinction between enthalpy and internal-energy of a system is due to external pressure-volume work carried out reversibly at constant gas pressure. The enthalpy change of the system, dH, at constant pressure, is the internal-energy change, dU, minus reversible pressure-volume work,
dH = dU - dVW Pressure-volume work, dVW, at constant pressure, is the gas pressure, p [Pa = J·m-3], times change of volume, dV [m3], dVW = -p·dV [J] The available work, deW, is distinguished from external total work, detW, [1] deW = detW - dVW The change of enthalpy of a system is due to internal and external changes, dH = diH + deH Since diH = 0 (first law of thermodynamics), the dH is balanced by exchange of heat, work, and matter, dH = (deQ + deW) + dmatH ; dp = 0 The exchange of matter is expressed in enthalpy equivalents with respect to a reference state (formation, f, or combustion, c). The value of dH in an open system, therefore, depends on the arbitrary choice of the reference state. In contrast, the terms in parentheses are the sum of all (total, t) partial energy transformations, dtH = (deQ + deW) A partial enthalpy change of transformation, dtrH, is distinguished from the total enthalpy change of all transformations, dtH, and from the enthalpy change of the system, dH. In a closed system, dH = dtH. The enthalpy change of transformation is the sum of the Gibbs energy (free energy) change of transformation, dtrG, and the bound energy change of transformation at constant temperature and pressure, dtrB = T·dS, dtrH = dtrG + dtrB |
| Entity | X | An entity of type X is something that can measured as an extensive quantity or counted as an elementary entity. The term entity with symbol X, therefore, has a general meaning, including but not limited to elementary entities UX. The distinction can be emphasized by using the term entity-type X, to avoid confusion of an entity X with the more restricted definition of elementary entity UX as a single countable object or event. |
| Equality | = | Physicochemical equality (symbol =) indicates in an equation not only numerical equivalence (symbol ≡), but an identity of the full meaning. |
| Equivalence | ≡ | Numerical equivalence (symbol ≡) indicates that two quantities are numerically equal, even if the full meaning may be different. For instance: 1 ≡ 1·1 and 1 ≡ 1/1. In contrast to ≡, the symbol = indicates physicochemical equality. |
| Ergodynamic efficiency | ε | The ergodynamic efficiency, ε (compare thermodynamic efficiency), is a power ratio between the output power and the (negative) input power of an energetically coupled process. Since power [W] is the product of a flow and the conjugated thermodynamic force, the ergodynamic efficiency is the product of an output/input flow ratio and the corresponding force ratio. The efficiency is 0.0 in a fully uncoupled system (zero output flow) or at level flow (zero output force). The maximum efficiency of 1.0 can be reached only in a fully (mechanistically) coupled system at the limit of zero flow at ergodynamic equilibrium. The ergodynamic efficiency of coupling between ATP production (DT phosphorylation) and oxygen consumption is the flux ratio of DT phosphorylation flux and oxygen flux (P»/O2 ratio) multiplied by the corresponding force ratio. Compare with the OXPHOS-coupling efficiency. |
| Ethanol | ethanol abs. |
Ethanol or ethyl alcohol, C2H6O or EtOH, is widely used in the laboratory, particularly as a solvent and cleaning agent. There are different grades of high purity ethanol. Up to a purity of 95.6 % ethanol can be separated from water by destillation. Higher concentrations than 95% require usage of additives that disrupt the azeotrope composition and allow further distillation. Ethanol is qualified as "absolute" if it contains no more than one percent water. Whenever 'ethanol abs.' is mentioned without further specification in published protocols, it refers to ≥ 99 % ethanol a.r. (analytical reagent grade).
|
| Ethylene glycol tetraacetic acid | EGTA | Ethylene glycol tetraacetic acid (EGTA) is a chelator for heavy metals, with high affinity for Ca2+ but low affinity for Mg2+. Sigma E 4378. |
| Etomoxir | Eto | Etomoxir (Eto; 2[6(4-chlorophenoxy)hexyl]oxirane-2-carboxylate) is an irreversible inhibitor of carnitine palmitoyltransferase I (CPT-I) on the outer face of the mitochondrial inner membrane. Eto inhibits fatty acid oxidation by blocking the formation of acyl carnitines from long-chain fatty acids which require the carnitine shuttle for transport into mitochondria. In contrast to long-chain fatty acids, the transport of short- and medium-chain fatty acids is carnitine-independent. |
| Euthanyl/Pentobarbitol | Euthanyl | I am often asked by reviewers to discuss the effects of pentobarbitol euthansia on mithochondrial function. Takaki 1997 JJP: This paper has been helpful in this discussion. (edit by Staples JF) |
| Events - DatLab | F4 | An event in DatLab is a defined point in time, labelled by a name (1 to 10 characters). An event applies to all plots of the selected O2k-Chamber. The event is shown by a vertical line in the graph and the label of the event is shown on the top (DatLab 6 and lower: on the bottom). The default name is the sequential number of the event. It is recommended to edit event labels with a minimum number of characters, and to explain the abbreviation in the 'Definition' box. The final concentration and titration volume can be entered into the corresponding boxes, if the event relates to the titration of a substance. A short comment can be entered to describe the event in detail.
Set events - Manual events are entered (real-time, connected to the O2k) by pressing [F4] at the time of the event (e.g. to indicate a manual titration into the chamber). An event belongs either to chamber A, chamber B, or both. Instrumental events are added automatically, e.g. when the stirrer (A or B) or illumination (both chambers) is switched on or off. After setting a new event the Edit event window pops up. Pressing F4 defines the time point of the event. Full attention can then be paid to the experiment. Edit the event later, as it is possible to insert an event at any chosen moment of the plotted record of the experiment by placing the cursor anywhere in the graph at the selected time point by pressing Ctrl and clicking the left mouse button. Edit event - Left click on the name of an existing event to open the Edit event window to edit or Delete event. In events obtained from a selected protocol, the entire sequence of consecutive events is defined with event names, definitions, concentrations and titration volumes. Name - Enter an event name of 1 to 10 characters. Short names (e.g. O instead of Open) are recommended. Comment - Further information can be entered into the text field. Select O2k-chamber A, B or both. The Event will be shown on plots for both or one selected chamber. »Protocol events |
| Exergy | E; various [J] | Exergy includes external and internal work. Exergy as the external work is defined in the First Law of thermodynamics as a specific form of energy. Exergy as the dissipated Gibbs or Helmholtz energy is the irreversibly dissipated (internal) loss of the potential of performing work as defined in the Second Law of Thermodynamics.
Changes of exergy dG plus bound energy yield the enthalpy change: dH = dG + T∙dS = dG + dB |
| Experimental code | F3 | An experimental code can be entered in the Sample window, containing up to 10 digits. |
| Experimental log - DatLab | Ctrl+F3 | Experimental log provides an automatically generated experimental protocol with detailed information about the O2k settings and calibrations, the Sample information and various Events. Time-dependent information can be viewed for a single chamber or both chambers. The filter can be selected for viewing minimum information, intermittent by default, or all information. The experimental log can be viewed and saved as a PDF file by clicking on [Preview]. |
| Export as CSV - DatLab | Ctrl-E | Export as CSV (*.csv) exports plots and events to a text file for further use in Excel and other programs. |
| External flow | Ie [MU·s-1] | External flows across the system boundaries are formally reversible. Their irreversible facet is accounted for internally as transformations in a heterogenous system (internal flows, Ii). |
| Extinction coefficient | ε | The extinction coefficient (ε) of a substance is the absorbance of a 1 µmolar concentration over a 1 cm pathlength and is wavelength-dependent. |
| FADH2 | FADH2 | FADH2 and FAD: see Flavin adenine dinucleotide. |
| FCCP | FCCP | FCCP (Carbonyl cyanide p-trifluoro-methoxyphenyl hydrazone, C10H5F3N4O) is a protonophore or uncoupler: added at uncoupler concentration Uc; c is the optimum uncoupler concentration in titrations to obtain maximum mitochondrial respiration in the noncoupled state of ET capacity. |
| FN | FN | FN is induced in mt-preparations by addition of NADH-generating substrates (N-pathway control state, or CI-linked pathway control) in combination with one or several fatty acids, which are supplied to feed electrons into the F-junction through fatty acyl CoA dehydrogenase (reduced form FADH2), to electron transferring flavoprotein (CETF), and further through the Q-junction to Complex III (CIII). FAO not only depends on electron transfer through the F-junction (which is typically rate-limiting), but simultaneously generates FADH2 and NADH and thus depends on N-junction throughput. Hence FAO can be inhibited completely by inhibition of Complex I (CI). This physiological substrate combination is required for partial reconstitution of TCA cycle function and convergent electron-input into the Q-junction, to compensate for metabolite depletion into the incubation medium. FS in combination exerts an additive effect of convergent electron flow in most types of mitochondria. |
| FNS | FNS | FNS is induced in mt-preparations by addition of NADH-generating substrates (N-pathway control state, or CI-linked pathway control) in combination with succinate (S-pathway control state; S- or CII-linked) and one or several fatty acids, which are supplied to feed electrons into the F-junction through fatty acyl CoA dehydrogenase (reduced form FADH2), to electron transferring flavoprotein (CETF), and further through the Q-junction to Complex III (CIII). FAO not only depends on electron transfer through the F-junction (which is typically rate-limiting), but simultaneously generates FADH2 and NADH and thus depends on N-junction throughput. Hence FAO can be inhibited completely by inhibition of Complex I (CI). This physiological substrate combination is required for partial reconstitution of TCA cycle function and convergent electron-input into the Q-junction, to compensate for metabolite depletion into the incubation medium. FNS in combination exerts an additive effect of convergent electron flow in most types of mitochondria. |
| FNSGp | FNSGp |
MitoPathway control state: FNSGp
SUIT protocol: SUIT-002 This substrate combination supports convergent electron flow to the Q-junction. |
| Faraday constant | F [C/mol] | The Faraday constant F links the electric charge [C] to amount [mol], and thus relates the electrical format e [C] to the molar format n [mol]. The Farady constant, F = e·NA = 96 485.33 C/mol, is the product of elementary charge, e = 1.602176634∙10-19 C/x, and the Avogadro constant, NA = 6.02214076∙1023 x/mol. The dimensionless unit [x] is not explicitely considered by IUPAC. |
| Fatty acid | FA | Fatty acids are carboxylic acids with a carbon aliphatic chain. The fatty acids can be divided by the length of this chain, being considered as short-chain (1–6 carbons), medium-chain (7–12 carbons) and long-chain and very long-chain fatty acids (>12 carbons).
Long-chain fatty acids must be bound to carnitine to enter the mitochondrial matrix, in a reaction that can be catalysed by carnitine acyltransferase. For this reason, long-chain fatty acids, such as palmitate (16 carbons) is frequently supplied to mt-preparations in the activated form of palmitoylcarnitine. Fatty acids with shorter chains, as octanoate (8 carbons) may enter the mitochondrial matrix, however, in HRR they are more frequently supplied also in the activated form, such as octanoylcarnitine. Once in the mitochondrial matrix, the fatty acid oxidation (FAO) occurs, generating acetyl-CoA, NADH and FADH2. In the fatty acid oxidation pathway control state electrons are fed into the F-junction involving the electron transferring flavoprotein (CETF). FAO cannot proceed without a substrate combination of fatty acids & malate, and inhibition of CI blocks FAO. Low concentration of malate, typically 0.1 mM, does not saturate the N-pathway; but saturates the F-pathway. |
| Fatty acid oxidation | FAO | Fatty acid oxidation is a multi-step process by which fatty acids are broken down in β-oxidation to generate acetyl-CoA, NADH and FADH2 for further electron transfer to CoQ. Whereas NADH is the substrate of CI, FADH2 is the substrate of electron-transferring flavoprotein complex (CETF) which is localized on the matrix face of the mtIM, and supplies electrons from FADH2 to CoQ. Before the ß-oxidation in the mitochondrial matrix, fatty acids (short-chain with 1-6, medium-chain with 7–12, long-chain with >12 carbon atoms) are activated by fatty acyl-CoA synthases (thiokinases) in the cytosol. For the mitochondrial transport of long-chain fatty acids the mtOM-enzyme carnitine palmitoyltransferase I (CPT-1; considered as a rate-limiting step in FAO) is required which generates an acyl-carnitine intermediate from acyl-CoA and carnitine. In the next step, an integral mtIM protein carnitine-acylcarnitine translocase (CACT) catalyzes the entrance of acyl-carnitines into the mitochondrial matrix in exchange for free carnitines. In the inner side of the mtIM, another enzyme carnitine palmitoyltransferase 2 (CPT-2) converts the acyl-carnitines to carnitine and acyl-CoAs, which undergo ß-oxidation in the mitochondrial matrix. Short- and medium-chain fatty acids do not require the carnitine shuttle for mitochondrial transport. Octanoate, but not palmitate, (eight- and 16-carbon saturated fatty acids) may pass the mt-membranes, but both are frequently supplied to mt-preparations in the activated form of octanoylcarnitine or palmitoylcarnitine. |
| Fatty acid oxidation pathway control state | F, FAO | In the fatty acid oxidation pathway control state (F- or FAO-pathway), one or several fatty acids are supplied to feed electrons into the F-junction through fatty acyl CoA dehydrogenase (reduced form FADH2), to electron transferring flavoprotein (CETF), and further through the Q-junction to Complex III (CIII). FAO not only depends on electron transfer through the F-junction (which is typically rate-limiting relative to the N-pathway branch), but simultaneously generates FADH2 and NADH and thus depends on N-junction throughput. Hence FAO can be inhibited completely by inhibition of Complex I (CI). In addition and independent of this source of NADH, the type N substrate malate is required at low concentration (0.1 mM) as a co-substrate for FAO in mt-preparations, since accumulation of Acetyl-CoA inhibits FAO in the absence of malate. Malate is oxidized in a reaction catalyzed by malate dehydrogenase to oxaloacetate (yielding NADH), which then stimulates the entry of Acetyl-CoA into the TCA cycle catalyzed by citrate synthase. Peroxysomal β-oxidation carries out few β-oxidation cycles, thus shortening very-long-chain fatty acids (>C20) for entry into mitochondrial β-oxidation. Oxygen consumption by peroxisomal acyl-CoA oxidase is considered as residual oxygen consumption rather than cell respiration. |
| File search - DatLab | Ctrl+F | File search yields a list of all files labelled by the experimental code in a selected directory . Click on the file to preview the experimental log. With File Search you can search in all folders and subfolders on your computer for DatLab files with a selected experimental code. The experimental code is entered in the DatLab file in the window "Experiment" ([F3]). When you click on a folder and press the button search, the DatLab file names will appear on the right window. Click on a DatLab file and further information (e.g. Sample information, Background information) will appear in the window below. |
| Flavin adenine dinucleotide | FAD, FADH2 | Flavin adenine dinucleotide, FAD and FADH2, is an oxidation-reduction prosthetic group (redox cofactor; compare NADH). FMN and FAD are the prosthetic groups of flavoproteins (flavin dehydrogenases). Type F substrates (fatty acids) generate FADH2, the substrate of electron transferring flavoprotein (CETF). Thus FADH2 forms a junction or funnel of electron transfer to CETF, the F-junction (compare N-junction, Q-junction), in the F-pathway control state. In contrast, FADH2 is not the substrate but the internal product of succinate dehydrogenase (CII). FAD is the oxidized (quinone) form, which is reduced to FADH2 (hydroquinone form) by accepting two electrons and two protons. |
| Flow | I [MU∙s-1] | In an isomorphic analysis, any form of flow, I is the advancement of a process per unit of time, expressed in a specific motive unit [MU∙s-1], e.g., ampere for electric flow or current [A≡C∙s-1], watt for heat flow [W≡J∙s-1], and for chemical flow the unit is [mol∙s-1]. Flow is an extensive quantity. The corresponding isomorphic forces are the partial exergy (Gibbs energy) changes per advancement [J∙MU-1], expressed in volt for electric force [V≡J∙C-1], dimensionless for thermal force, and for chemical force the unit is [J∙mol-1], which deserves a specific acronym ([Jol]) comparable to volt. |
| Flux | J | Flux, J, is a specific quantity. Flux is flow, I [MU·s-1 per system] (an extensive quantity), divided by system size. Flux (e.g., oxygen flux) may be volume-specific (flow per volume [MU·s-1·L-1]), mass-specific (flow per mass [MU·s-1·kg-1]), or marker-specific (e.g. flow per mtEU). The motive unit [MU] of chemical flow or flux is the advancement of reaction [mol] in the chemical format. |
| Flux / Slope | J | Flux / Slope is the time derivative of the signal. In DatLab, Flux / Slope is the name of the pull-down menu for (1) normalization of flux (chamber volume-specific flux, sample-specific flux or flow, or flux control ratios), (2) flux baseline correction, (3) Instrumental background oxygen flux, and (4) flux smoothing, selection of the scaling factor, and stoichiometric normalization using a stoichiometric coefficient. Before changing the normalization of flux from volume-specific flux to sample-specific flux or flow, or flux control ratios, please be sure to use the standard Layout 04a (Flux per volume) or 04b (Flux per volume overlay). When starting with the instrumental standard Layouts 1-3, which display the O2 slope negative, the sample-specific flux or flow, or flux control ratios will not be automatically background corrected. To obtain the background corrected specific flux or flux control ratios, it is needed to tick the background correction in the lower part of the slope configuration window. Background correction is especially critical when performing measurements in a high oxygen regime or using samples with a low respiratory flux or flow. |
| Flux baseline correction | bc | Flux baseline correction provides the option to display the plot and all values of the flux (or flow, or flux control ratio) as the total flux, J, minus a baseline flux, J0.
JV(bc) = JV - JV0 JV = (dc/dt) · ν-1 · SF - J°VFor the oxygen channel, JV is O2 flux per volume [pmol/(s·ml)] (or volume-specific O2 flux), c is the oxygen concentration [nmol/ml = µmol/l = µM], dc/dt is the (positive) slope of oxygen concentration over time [nmol/(s · ml)], ν-1 = -1 is the stoichiometric coefficient for the reaction of oxygen consumption (oxygen is removed in the chemical reaction, thus the stoichiometric coefficient is negative, expressing oxygen flux as the negative slope), SF=1,000 is the scaling factor (converting units for the amount of oxygen from nmol to pmol), and J°V is the volume-specific background oxygen flux (Instrumental background oxygen flux). Further details: Flux / Slope. |
| Flux control efficiency | jZ-Y | Flux control efficiencies express the control of respiration by a metabolic control variable, X, as a fractional change of flux from YX to ZX, normalized for ZX. ZX is the reference state with high (stimulated or un-inhibited) flux; YX is the background state at low flux, upon which X acts.
Complementary to the concept of flux control ratios and analogous to elasticities of metabolic control analysis, the flux control efficiency of X upon background YX is expressed as the change of flux from YX to ZX normalized for the reference state ZX. » MiPNet article |
| Flux control ratio | FCR | Flux control ratios FCRs are ratios of oxygen flux in different respiratory control states, normalized for maximum flux in a common reference state, to obtain theoretical lower and upper limits of 0.0 and 1.0 (0 % and 100 %). For a given protocol or set of respiratory protocols, flux control ratios provide a fingerprint of coupling and substrate control independent of (1) mt-content in cells or tissues, (2) purification in preparations of isolated mitochondria, and (3) assay conditions for determination of tissue mass or mt-markers external to a respiratory protocol (CS, protein, stereology, etc.). FCR obtained from a single respirometric incubation with sequential titrations (sequential protocol; SUIT protocol) provide an internal normalization, expressing respiratory control independent of mitochondrial content and thus independent of a marker for mitochondrial amount. FCR obtained from separate (parallel) protocols depend on equal distribution of subsamples obtained from a homogenous mt-preparation or determination of a common mitochondrial marker. |
| Force | F; dmFX; ΔtrFX [J·MU-1] | Force is an intensive quantity. The product of force times advancement is the work (exergy) expended in a process or transformation. Force times flow is power [W].
|
| Free activity | αX [MU·m-3] | Free activity αX [MU·m-3] is pressure divided by isomorphic force. In the chemical amount format, αX is expressed in units of concentration of X [mol·L-1]. αX is the local concentration in a concentration gradient. If the concentration gradient is collapsed to a boundary of zero thickness in a compartmental system, αX reflects the singularity in the transition between the two phases or compartments. |
| French Group of Bioenergetics | FGoB | The French Group of Bioenergetics... |
| Fumarase | FH | Fumarase or fumarate hydratase (FH) is an enzyme of the tricarboxylic acid cycle catalyzing the equilibrium reaction between fumarate and malate. Fumarase is found not only in mitochondria, but also in the cytoplasm of all eukaryotes. |
| GM-pathway control state | GM | GM: Glutamate & Malate.
MitoPathway control state: NADH electron transfer-pathway state The GM-pathway control state (glutamate-malate pathway control state) is established when glutamate&malate are added to isolated mitochondria, permeabilized cells and other mitochondrial preparations. Glutamate and transaminase are responsible for the metabolism of oxaloacetate, comparable to the metabolism with acetyl-CoA and citrate synthase. |
| GMS-pathway control state | GMS | GMS: Glutamate & Malate & Succinate.
MitoPathway control: NS Transaminase catalyzes the reaction from oxaloacetate to 2-oxoglutarate, which then establishes a cycle without generation of citrate. OXPHOS is higher with GS (CI&II) compared to GM (CI) or SRot (CII). This documents an additive effect of convergent CI&II electron flow to the Q-junction, with consistent results obtained with permeabilized muscle fibres and isolated mitochondria (Gnaiger 2009). |
| Gas constant | R [J·mol-1·K-1] | The gas constant, R = 8.314462618 J·mol-1·K-1, has the SI unit for energy per amount per temperature. R is primarily known from the ideal gas equation, pV = nRT or p = cRT. Therefore, RT is the ratio of pressure p and concentration c.
R = f·F, the electrochemical constant f times the Faraday constant F. R = k·NA, the Boltzmann constant k times the Avogadro constant NA. |
| Gibbs energy | G [J] | Gibbs energy G [J] is exergy which cannot be created internally (subscript i), but in contrast to internal-energy (diU/dt = 0) is not conserved but is dissipated (diG/dt < 0) in irreversible energy transformations at constant temperature and (barometric) pressure, T,p. Exergy is available as work in reversible energy transformations (100 % efficiency), and can be partially conserved when the exergonic transformation is coupled to an endergonic transformation. |
| Glucose | Glc | Glucose, also known as D-glucose or dextrose, is a monosaccharide and an important carbohydrate in biology. Cells use it as the primary source of energy and a metabolic intermediate. |
| Glutamate | G | Glutamic acid, C5H9NO4, is an amino acid which occurs under physiological conditions mainly as the anion glutamate-, G, with pKa1 = 2.1, pKa2 = 4.07 and pKa3 = 9.47. Glutamate&malate is a substrate combination supporting an N-linked pathway control state, when glutamate is transported into the mt-matrix via the glutamate-aspartate carrier and reacts with oxaloacetate in the transaminase reaction to form aspartate and oxoglutarate. Glutamate as the sole substrate is transported by the electroneutral glutamate-/OH- exchanger, and is oxidized in the mitochondrial matrix by glutamate dehydrogenase to α-ketoglutarate (2-oxoglutarate), representing the glutamate-anaplerotic pathway control state. Ammonia (the byproduct of the reaction) passes freely through the mitochondrial membrane. |
| Glutamate dehydrogenase | mtGDH | Glutamate dehydrogenase, located in the mitochondrial matrix (mtGDH), is an enzyme that converts glutamate to α-ketoglutarate [1]. mtGDH is not part of the TCA cycle, but is involved in glutaminolysis as an anaplerotic reaction. |
| Glutamate-anaplerotic pathway control state | G | G: Glutamate is an anaplerotic NADH-linked type 4 substrate (N). When supplied as the sole fuel substrate in the glutamate-anaplerotic pathway control state, G is transported by the electroneutral glutamate-/OH- exchanger, and is oxidised via mt-glutamate dehydrogenase in the mitochondrial matrix. The G-pathway plays an important role in glutaminolysis. |
| Glycerophosphate | Gp | Glycerophosphate (synonym: α-glycerophosphate; glycerol-3-phosphate; C3H9O6P) is an organophosphate and it is a component of glycerophospholipids. The mitochondrial Glycerophosphate dehydrogenase Complex oxidizes glycerophosphate to dihydroxyacetone phosphate and feeds electrons directly to ubiquinone. |
| Glycerophosphate dehydrogenase Complex | CGpDH | Glycerophosphate dehydrogenase complex (CGpDH) is a Complex of the electron transfer-pathway localized at the outer face of the mt-inner membrane. CGpDH is thus distinguished from cytosolic GpDH. CGpDH oxidizes glycerophosphate to dihydroxyacetone phosphate and feeds two electrons into the Q-junction, thus linked to an ET pathway level 3 control state. |
| Glycerophosphate pathway control state | Gp | The glycerophosphate pathway control state (Gp) is an ET-pathway level 3 control state, supported by the fuel substrate glycerophosphate and electron transfer through glycerophosphate dehydrogenase Complex into the Q-junction. The glycerolphosphate shuttle represents an important pathway, particularly in liver and blood cells, of making cytoplasmic NADH available for mitochondrial oxidative phosphorylation. Cytoplasmic NADH reacts with dihydroxyacetone phosphate catalyzed by cytoplasmic glycerophos-phate dehydrogenase. On the outer face of the inner mitochondrial membrane, mitochondrial glycerophosphate dehydrogenase oxidises glycerophosphate back to dihydroxyacetone phosphate, a reaction not generating NADH but reducing a flavin prosthesic group. The reduced flavoprotein donates its reducing equivalents to the electron transfer-pathway at the level of CoQ. |
| Glycerophosphate shuttle | Gp shuttle | The glycerophosphate shuttle makes cytoplasmic NADH available for mitochondrial oxidative phosphorylation. Cytoplasmic NADH reacts with dihydroxyacetone phosphate catalyzed by cytoplasmic glycerophosphate dehydrogenase. On the outer face of the inner mitochondrial membrane, glycerophosphate dehydrogenase complex (mitochondrial glycerophosphate dehydrogenase) oxidizes glycerophosphate back to dihydroxyacetone phosphate, a reaction not generating NADH but reducing a flavin prosthesic group. The reduced flavoprotein transfers its reducing equivalents into the Q-junction, thus representing a ET pathway level 3 control state. |
| H2DCFDA | DCF, H2-DCF | H2DCFDA (dichlorodihydrofluorescein diacetate) is a cell permeant fluorescent probe that has been used as an indicator of ROS presence. It is a reduced form of fluorescein that does not present fluorescence. After entry in the cell, it suffers deacetylation by intracellular esterases, and upon oxidation it is converted to dichlorofluorescein (excitation wavelength ~492–495 nm, emission ~517–527 nm). It may be oxidised by hydrogen peroxide, hydroxyl radical, hypochlorite anion, nitric oxide, peroxyl radical, peroxynitrite, singlet oxygen and superoxide. Has been used as a general indicator of ROS by fluorescence microscopy. |
| HPTS | HPTS | 8-Hydroxypyrene-1,3,6-trisulfonic acid trisodium salt (HPTS) is a ratiometric pH fluorophore; pKa = 7.3. Relative molecular mass: Mr = 524.39 |
| Harmonized European norm | EN-norm | Harmonized European norms are norms valid for all members of the European Union. They are mandatory parts of the individual national collections of norms. |
| Harmonized SUIT protocols | H-SUIT | Harmonized SUIT protocols (H-SUIT) are designed to include cross-linked respiratory states. When performing harmonized SUIT protocols in parallel, measurements of cross-linked respiratory states can be statistically evaluated as replicates across protocols. Additional information is obtained on respiratory coupling and substrate control by including respiratory states that are not common (not cross-linked) across the harmonized protocols. |
| Healthy reference population | HRP | A healthy reference population, HRP, establishes the baseline for the relation between body mass and height in healthy people of zero underweight or overweight, providing a reference for evaluation of deviations towards underweight or overweight and obesity. The WHO Child Growth Standards (WHO-CGS) on height and body mass refer to healthy girls and boys from Brazil, Ghana, India, Norway, Oman and the USA. The Committee on Biological Handbooks compiled data on height and body mass of healthy males from infancy to old age (USA), published before emergence of the fast-food and soft-drink epidemic. Four allometric phases are distinguished with distinct allometric exponents. At heights above 1.26 m/x the allometric exponent is 2.9, equal in women and men, and significantly different from the exponent of 2.0 implicated in the body mass index, BMI [kg/m2]. |
| Heat | Q, Qth [J] | Heat is a form of energy [J]. The relationship between heat and work provides the foundation of thermodynamics, which describes transformations from an initial to a final state of a system. In energy transformations heat may pass through the boundary of the system, at an external heat flow of deQ/dt. |
| Height of humans | h [m]; H [m·x-1] | The height of humans, h, is given in SI units in meters [m]. Humans are countable objects, and the symbol and unit of the number of objects is N [x]. The average height of N objects is, H = h/N [m/x], where h is the heights of all N objects measured on top of each other. Therefore, the height per human has the unit [m·x-1] (compare body mass [kg·x-1]). Without further identifyer, H is considered as the standing height of a human, measured without shoes, hair ornaments and heavy outer garments. |
| Hexokinase | HK | The hexokinase catalyzes the phosphorylation of D-glucose at position 6 by ATP to yield D-glucose 6-phosphate as well as the phosphorylation of many other hexoses like D-fructose, D-mannose, D-glucosamine. |
| High-resolution respirometry | HRR | High-resolution respirometry, HRR, is the state-of-the-art approach in mitochondria and cell research to measure respiration in various types of mitochondrial preparations and living cells combined with MultiSensor modules.
Mitochondrial function and dysfunction have gained increasing interest, reflecting growing awareness of the fact that mitochondria play a pivotal role in human health and disease. HRR combines instrumental accuracy and reliability with the versatility of applicable protocols, allowing practically unlimited addition and combination of substrates, inhibitors, and uncouplers using the Oroboros O2k-technology. Substrate-uncoupler-inhibitor titration (SUIT) protocols allow the interrogation of numerous mitochondrial pathway and coupling states in a single respirometric assay. Mitochondrial respiratory pathways may be analyzed in detail to evaluate even minor alterations in respiratory coupling and pathway control patterns. The O2k-technology provides sole source instruments, with no other available instrument meeting its specifications for high-resolution respirometry. Technologically, HRR is based on the Oroboros O2k-technology, combining optimized chamber design, application of oxygen-tight materials, electrochemical sensors, Peltier-temperature control, and specially developed software features (DatLab) to obtain the unique sensitive and quantitative resolution of oxygen concentration and oxygen flux, with both, a closed-chamber or open-chamber mode of operation (TIP2k). Standardized calibration of the polarographic oxygen sensor (static sensor calibration), calibration of the sensor response time (dynamic sensor calibration), and evaluation of instrumental background oxygen flux (systemic flux compensation) provide the experimental basis for high accuracy of quantitative results and quality control in HRR. HRR can be extended for MultiSensor analysis by using the O2k-Fluo Smart-Module. Smart Fluo-Sensors are integrated into the O2k to measure simultaneously fluorometric signals using specific fluorophores. Potentiometric modules are available with ion-selective electrodes (pH, TPP+). The PB-Module extends HRR to PhotoBiology with accurate control of the light intensity and measurement of photosynthesis. The O2k and the NextGen-O2k support all these O2k-Modules. The NextGen-O2k all-in-one, however, is unique in supporting Q-Redox and NADH-Redox Modules. |
| Horseradish peroxidase | HRP | Horseradish peroxidase readily combines with hydrogen peroxide (H2O2) and the resultant [HRP-H2O2] complex can oxidize a wide variety of hydrogen donors. |
| Hydride | H- | The hydride anion is the species H−. |
| Hydrogen | H2 | Molecular hydrogen H2 is a constituent of the air with a volume fraction of 0.00005. It is a colorless and odorless gas with a molecular mass of 2.016. Its pharmacological potential and effects on mitochondrial metabolism are discussed in various publications without complete evidence on the underlying mechanisms. |
| Hydrogen ion | H+ | The terms hydrogen ion H+ and proton, p or p+, are used synonymously in chemistry. A hydrogen ion is a positively charged molecule. In particle physics, however, a proton is a submolecular and subatomic particle with a positive electric charge. The H+ ion has no electrons and is a bare charge with only about 1/64 000 of the radius of a hydrogen atom. Free H+ is extremely reactive, with an extremely short lifetime in aqueous solutions. There H+ forms the hydronium ion H3O+, which in turn is further solvated by water molecules in clusters such as H5O2+ and H9O4+. The transfer of H+ in an acid–base reaction is referred to as proton transfer. The acid is the H+ donor and the base is the H+ acceptor. |
| Hydrogen peroxide | H2O2 | Hydrogen peroxide, H2O2 or dihydrogen dioxide, is one of several reactive oxygen intermediates generally referred to as reactive oxygen species (ROS). It is formed in various enzyme-catalyzed reactions (e.g., superoxide dismutase) with the potential to damage cellular molecules and structures. H2O2 is dismutated by catalase to water and oxygen. H2O2 is produced as a signaling molecule in aerobic metabolism and passes membranes more easily compared to other ROS. |
| Hydrogen sulfide | H2S | Hydrogen sulfide (H2S) is involved in signaling and may have have further biological importance. |
| Hydrogenion flux | JH+ | Volume-specific hydrogenion flux or H+ flux is measured in a closed system as the time derivative of H+ concentration, expressed in units [pmol·s-1·mL-1]. H+ flux can be measured in an open system at steady state, when any acidification of the medium is compensated by external supply of an equivalent amount of base. The extracellular acidification rate (ECAR) is the change of pH in the incubation medium over time, which is zero at steady state. Volume-specific H+ flux is comparable to volume-specific oxygen flux [pmol·s-1·mL-1], which is the (negative) time derivative of oxygen concentration measured in a closed system, corrected for instrumental and chemical background. pH is the negative logarithm of hydrogen ion activity. Therefore, ECAR is of interest in relation to acidification issues in the incubation buffer or culture medium. The physiologically relevant metabolic H+ flux, however, must not be confused with ECAR. |
| Hydron | H+ | 'Hydron is the general name for the cation H+ used without regard to the nuclear mass of the hydrogen entity (H is the hydro group), either for H in its natural abundance or without distinction between the isotopes. |
| Hydronium ion | H3O+ | H+ forms the hydronium ion H3O+, which in turn is further solvated by water molecules in clusters such as H5O2+ and H9O4+. |
| Hydroxycinnamate | Hci | Hydroxycinnamate (alpha-cyano-4-hydroxycinnamic acid) is an inhibitor of the pyruvate carrier (0.65 mM). Above 10 mM pyruvate, hydroxycinnamate cannot inhibit respiration from pyruvate, since the weak pyruvic acid can pass the inner mt-membrane in non-dissociated form. |
| Hyperoxia | hyperox | Hyperoxia is defined as environmental oxygen pressure above the normoxic reference level. Cellular and intracellular hyperoxia is imposed on isolated cells and isolated mitochondria at air-level oxygen pressures which are higher compared to cellular and intracellular oxygen pressures under tissue conditions in vivo. Hyperoxic conditions may impose oxidative stress and may increase maximum aerobic performance. |
| Hypoxia | hypox | Hypoxia (hypox) is defined in respiratory physiology as the state when insufficient O2 is available for respiration, compared to environmental hypoxia defined as environmental oxygen pressures below the normoxic reference level. Three major categories of hypoxia are (1) environmental hypoxia, (2) physiological tissue hypoxia in hyperactivated states (e.g. at VO2max) with intracellular oxygen demand/supply balance at steady state in tissues at environmental normoxia, compared to tissue normoxia in physiologically balanced states, and (3) pathological tissue hypoxia including ischemia and stroke, anaemia, chronic heart disease, chronic obstructive pulmonary disease, severe COVID-19, and obstructive sleep apnea. Pathological hypoxia leads to tissue hypoxia and heterogenous intracellular anoxia. Clinical oxygen treatment ('environmental hyperoxia') may not or only partially overcome pathological tissue hypoxia. |
| IRDiRC | IRDiRC |  The International Rare Diseases Research Consortium (IRDiRC) teams up researchers and organizations investing in rare diseases research in order to achieve two main objectives by the year 2020, namely to deliver 200 new therapies for rare diseases and means to diagnose most rare diseases. The International Rare Diseases Research Consortium (IRDiRC) teams up researchers and organizations investing in rare diseases research in order to achieve two main objectives by the year 2020, namely to deliver 200 new therapies for rare diseases and means to diagnose most rare diseases. |
| ISO 10012:2003 Measurement management systems | ISO 10012:2003 | ISO 10012:2003 Measurement management systems — Requirements for measurement processes and measuring equipment: An effective measurement management system ensures that measuring equipment and measurement processes are fit for their intended use and is important in achieving product quality objectives and managing the risk of incorrect measurement results. The objective of a measurement management system is to manage the risk that measuring equipment and measurement processes could produce incorrect results affecting the quality of an organization’s product. The methods used for the measurement management system range from basic equipment verification to the application of statistical techniques in the measurement process control. |
| ISO 13528:2015 Statistical methods for use in proficiency testing by interlaboratory comparison | ISO 13528:2015 | ISO 13528:2015 Statistical methods for use in proficiency testing by interlaboratory comparison: Proficiency testing involves the use of interlaboratory comparisons to determine the performance of participants (which may be laboratories, inspection bodies, or individuals) for specific tests or measurements, and to monitor their continuing performance. There are a number of typical purposes of proficiency testing ISO/IEC 17043:2010. These include the evaluation of laboratory performance, the identification of problems in laboratories, establishing effectiveness and comparability of test or measurement methods, the provision of additional confidence to laboratory customers, validation of uncertainty claims, and the education of participating laboratories. The statistical design and analytical techniques applied must be appropriate for the stated purpose(s). |
| ISO 15189:2012 Medical laboratories — Particular requirements for quality and competence | ISO 15189:2012 | ISO 15189:2012 Medical laboratories — Particular requirements for quality and competence: This International Standard is for use by medical laboratories in developing their quality management systems and assessing their own competence, and for use by accreditation bodies in confirming or recognising the competence of medical laboratories. While this International Standard is intended for use throughout the currently recognised disciplines of medical laboratory services, those working in other services and disciplines could also find it useful and appropriate. |
| ISO 17511:2003 In vitro diagnostic medical devices | ISO 17511:2003 | ISO 17511:2003 In vitro diagnostic medical devices -- Measurement of quantities in biological samples -- Metrological traceability of values assigned to calibrators and control materials: For measurements of quantities in laboratory medicine, it is essential that the quantity is adequately defined and that the results reported to the physicians or other health care personel and patients are adequately accurate (true and precise) to allow correct medical interpretation and comparability over time and space. |
| ISO 9001:2015 Quality management systems - requirements | ISO 9001:2015 | ISO 9001:2015 Quality management systems - requirements: The adoption of a quality management system is a strategic decision for an organization that can help to improve its overall performance and provide a sound basis for sustainable development initiatives. Consistently meeting requirements and addressing future needs and expectations poses a challenge for organizations in an increasingly dynamic and complex environment. To achieve this objective, the organization might find it necessary to adopt various forms of improvement in addition to correction and continual improvement, such as breakthrough change, innovation and re-organization. |
| ISO/IEC 17025:2005 Competence of testing and calibration laboratories | ISO/IEC 17025:2005 | ISO/IEC 17025:2005 General requirements for the competence of testing and calibration laboratories: The use of this International Standard will facilitate cooperation between laboratories and other bodies, and assist in the exchange of information and experience, and in the harmonization of standards and procedures. This International Standard specifies the general requirements for the competence to carry out tests and/or calibrations, including sampling. It covers testing and calibration performed using standard methods, non-standard methods, and laboratory-developed methods. |
| ISO/IEC 17043:2010 General requirements for proficiency testing | ISO/IEC 17043:2010 | ISO/IEC 17043:2010 Conformity assessment — General requirements for proficiency testing: The use of interlaboratory comparisons is increasing internationally. This International Standard provides a consistent basis to determine the competence of organizations that provide proficiency testing. |
| Illumination | F10 | The chambers of the Oroboros O2k are illuminated by an internal LED. The illumination is switched on and off in DatLab during the experiment by pressing [F10]. This illumination must be distinguished from light introduced into the chambers by LEDs for the purpose of spectrophotometric and fluorometric measurements. For these, the internal illumination must be switched off. |
| Illumination on/off | F10 | The illumination in both chambers is switched on/off. |
| Impact factor | IF | Impact factor is a measure of a scientific journal's citations per publication. The Journal Citation Reports, maintained by Clarivate Analytics, provides the calculated impact factors. The IF is frequently used as an indicator of a journal's importance or prestige, which is nowadays increasingly contested. |
| Improvement score | RIS | The relative improvement score, RIS, provides a measure of improvement of a trait from a value measured at baseline, B, to a value measured after treatment, T, expressing the total improvement, T-B, in relation to the theoretical scope of improvement and the level of the trait observed at baseline. RIS incorporates the concept of diminishing returns and consideres maintaining a high value of a trait as an improvement relative to the potential loss. |
| In vitro diagnostic medical device | IVD | A medical device is an in vitro diagnostic medical device (IVD) if it is a reagent, calibrator, control material, kit, specimen receptacle, software, instrument, apparatus, equipment or system, whether used alone or in combination with other diagnostic goods for in vitro use. |
| Inorganic phosphate | Pi | Inorgnic phosphate (Pi) is a salt of phosphoric acid. In solution near physiological pH, the species HPO42- and H2PO4- dominate. See also: Phosphate carrier (Pic). |
| Internal flow | Ii [MU·s-1] | Within the system boundaries, irreversible internal flows, Ii,—including chemical reactions and the dissipation of internal gradients of heat and matter—contribute to internal entropy production, diS/dt. In contrast, external flows, Ie, of heat, work, and matter proceed reversibly across the system boundaries (of zero thickness). Flows are expressed in various formats per unit of time, with corresponding motive units [MU], such as chemical [mol], electrical [C], mass [kg]. Flow is an extensive quantity, in contrast to flux as a specific quantity. |
| Internal-energy | U [J] | Internal-energy, U [J], can neither be destroyed nor created (first law of thermodynamics: diU/dt = 0). Note that internal (subscript i), as opposed to external (subscript e), must be distinguished from "internal-energy", U, which contrasts with "Helmholtz energy", A, as enthalpy, H, contrasts with Gibbs energy, G. |
| International Mito Patients (IMP) | IMP |  The International Mito Patients is a network of national patient organizations involved in mitochondrial disease. Mitochondrial disease is a rare disease with a limited number of patients per country. The national patient organizations which are a member of IMP each are active and powerful in their own countries. By joining forces IMP can represent a large group of patients and as such be their voice on an international level. The International Mito Patients is a network of national patient organizations involved in mitochondrial disease. Mitochondrial disease is a rare disease with a limited number of patients per country. The national patient organizations which are a member of IMP each are active and powerful in their own countries. By joining forces IMP can represent a large group of patients and as such be their voice on an international level. |
| International Society for Mountain Medicine | ISMM | The International Society for Mountain Medicine is an interdisciplinary society comprising about xx members worldwide. Its purpose is .. |
| International Society on Oxygen Transport to Tissue | ISOTT | The International Society on Oxygen Transport to Tissue is an interdisciplinary society comprising about 250 members worldwide. Its purpose is to further the understanding of all aspects of the processes involved in the transport of oxygen from the air to its ultimate consumption in the cells of the various organs of the body. Founded in 1973, the society has been the leading platform for the presentation of many of the technological and conceptual developments within the field both at the meetings themselves and in the proceedings of the society. |
| International Standard Serial Number | ISSN | The International Standard Serial Number, ISSN, is a code used to identify periodical publications, independent of which media are used (print and/or electronic). - Bioenergetics Communications, BEC: ISSN 2791-4690 |
| International System of Units | SI | The International System of Units (SI) is the modern form of the metric system of units for use in all aspects of life, including international trade, manufacturing, security, health and safety, protection of the environment, and in the basic science that underpins all of these. The system of quantities underlying the SI and the equations relating them are based on the present description of nature and are familiar to all scientists, technologists and engineers. The definition of the SI units is established in terms of a set of seven defining constants. The complete system of units can be derived from the fixed values of these defining constants, expressed in the units of the SI. These seven defining constants are the most fundamental feature of the definition of the entire system of units. These particular constants were chosen after having been identified as being the best choice, taking into account the previous definition of the SI, which was based on seven base units, and progress in science (p. 125). |
| International Union of Pure and Applied Chemistry, IUPAC | IUPAC | The International Union of Pure and Applied Chemistry (IUPAC) celebrated in 2019 the 100th anniversary, which coincided with the International Year of the Periodic Table of Chemical Elements (IYPT 2019). IUPAC {Quote} notes that marking Mendeleev's achievement will show how the periodic table is central to connecting cultural, economic, and political dimensions of global society “through a common language” {end of Quote} (Horton 2019). 2019 is proclaimed as the International Year of the Periodic Table of Chemical Elements (IYPT 2019). For a common language in mitochondrial physiology and bioenergetics, the IUPAC Green book (Cohen et al 2008) is a most valuable resource, which unfortunately is largely neglected in bioenergetics textbooks. Integration of open systems and non-equilibrium thermodynamic approaches remains a challenge for developing a common language (Gnaiger 1993; BEC 2020.1). |
| International oxygraph course | IOC | International Oxygraph Course (IOC), see O2k-Workshops. |
| Internationale Gesellschaft fuer Regenerative Mitochondrien-Medizin | IGRMM e.V. | Organizer of |
| Intracellular oxygen | pO2,i | Physiological, intracellular oxygen pressure is significantly lower than air saturation under normoxia, hence respiratory measurements carried out at air saturation are effectively hyperoxic for cultured cells and isolated mitochondria. |
| Ionomycin | Imy | Ionomycin (Imy) is a ionophore used to raise intracellular [Ca2+]. |
| Isocitrate dehydrogenase | IDH | Isocitrate dehydrogenase forms 2-oxoglutarate from isocitrate in the TCA cycle. |
| Isolated mitochondria | imt | Isolated mitochondria, imt, are mitochondria separated from a tissue or cells by breaking the plasma membranes and attachments to the cytoskeleton, followed by centrifugation steps to separate the mitochondria from other components. |
| Japanese Society of Mitochondrial Research and Medicine | J-mit | The Japanese Society of Mitochondrial Research and Medicine (J-mit) was founded to share the latest knowledge on mitochondrial research. J-mit is the biggest Asian society of mitochondrial research and medicine and is a member of ASMRM. |
| Jmax | Jmax | Jmax is the maximum pathway flux (e.g. oxygen flux) obtained at saturating substrate concentration. Jmax is a function of metabolic state. In hyperbolic ADP or oxygen kinetics, Jmax is calculated by extrapolation of the hyperbolic function, with good agreement between the calculated and directly measured fluxes, when substrate levels are >20 times the c50 or p50. |
| Kelvin | K | The kelvin, symbol K, is the SI unit of thermodynamic temperature. It is defined by taking the fixed numerical value of the Boltzmann constant k to be 1.380 649 × 10−23 when expressed in the unit J x-1 K−1. |
| Kilogram | kg | The kilogram, symbol kg, is the SI unit of mass. It is defined by taking the fixed numerical value of the Planck constant h to be 6.626 070 15 × 10−34 when expressed in the unit J s, which is equal to kg m2 s−1, where the meter and the second are defined in terms of c and ΔνCs. |
| Korean Society of Mitochondrial Research and Medicine | KSMRM | The Korean Society of Mitochondrial Research and Medicine (KSMRM) is a member of ASMRM. |
| L/E coupling-control ratio | L/E | |
| L/P coupling-control ratio | L/P | |
| L/R coupling-control ratio | L/R | |
| LEAK respiration | L | |
| LEAK state with ATP | L(T) | |
| LEAK state with oligomycin | L(Omy) | |
| LEAK state without adenylates | L(n) | |
| Lactate dehydrogenase | LDH | Lactate dehydrogenase is a glycolytic marker enzyme in the cytosol, regenerating NAD+ from NADH and pyruvate, forming lactate. |
| Length | l [m] | Length l is an SI base quantity with SI base unit meter m. Quantities derived from length are area A [m2] and volume V [m3]. Length is an extensive quantity, increasing additively with the number of objects. The term 'height' h is used for length in cases of vertical position (see height of humans). Length of height per object, LUX [m·x-1] is length per unit-entity UX, in contrast to lentgth of a system, which may contain one or many entities, such as the length of a pipeline assembled from a number NX of individual pipes. Length is a quantity linked to direct sensory, practical experience, as reflected in terms related to length: long/short (height: tall/small). Terms such as 'long/short distance' are then used by analogy in the context of the more abstract quantity time (long/short duration). |
| Level flow | E | |
| Light-emitting diode | LED | A light-emitting diode (LED) is a light source (semiconductor), used in many every-day applications and specifically in fluorometry. LEDs are available for specific spectral ranges across wavelengths in the visible, ultraviolet, and infrared range. |
| Light-enhanced dark respiration | LEDR | Light-enhanced dark respiration LEDR is a sharp (negative) maximum of dark respiration in plants in response to illumination, measured immediately after switching off the light. LEDR is supported by respiratory substrates produced during photosynthesis and closely reflects light-enhanced photorespiration (Xue et al 1996). Based on this assumption, the total photosynthetic oxygen flux TP is calculated as the sum of the measured net photosynthetic oxygen flux NP plus the absolute value of LEDR. |
| Limiting oxygen pressure | pl | The limiting oxygen pressure, pl, is defined as the partial oxygen pressure, pO2, below which anaerobic catabolism is activated to contribute to total ATP generation. The limiting oxygen pressure, pl, may be substantially lower than the critical oxygen pressure, pc, below which aerobic catabolism (respiration or oxygen consumption) declines significantly. |
| Limiting pO2 | plim | In the transition from aerobic to anaerobic metabolism, there is a limiting pO2, plim, below which anaerobic energy flux is switched on and CR ratios become more exothermic than the oxycaloric equivalent. plim may be significanlty below the critical pO2. |
| Living Communications | LC | With Living Communications, Bioenergetics Communications (BEC) takes the next step from pre-print to re-print. The concept of Living Communications pursues a novel culture of scientific communication, addressing the conflict between long-term elaboration and validation of results versus sharing without delay improved methods and preliminary findings. Following the preprint concept, updates may be posted on the BEC website of the resource publication. Updated versions of Living Communications are submitted for Open Peer Review with full traceability. In contrast to static papers, evolution of Living Communications is more resourceful and efficient than a ‘new’ publication. Living Communications provide a pathway along the scientific culture of lively debate towards tested and trusted milestones of research, from pre-print to re-print, from initial steps to next steps. |
| Living cells | ce | Cell viability in living cells should be >95 % for various experimental investigations, including cell respirometry. Viable cells (vce) are characterized by an intact plasma membrane barrier function. The total cell count (Nce) is the sum of viable cells (Nvce) and dead cells (Ndce). In contrast, the plasma membrane can be permeabilized selectively by mild detergents (digitonin), to obtain the mt-preparation of permeabilized cells used for cell ergometry. Living cells are frequently labelled as intact cells in the sense of the total cell count, but intact may suggest dual meanings of viable or unaffected by a disease or mitochondrial injury. |
| MITOEAGLE in MitoGlobal | MitoEAGLE | The objective of the MitoEAGLE network is to improve our knowledge on mitochondrial function in health and disease related to Evolution, Age, Gender, Lifestyle and Environment. |
| Magnesium Green | MgG | Magnesium Green (MgG) is an extrinsic fluorophore that fluoresces when bound to Mg2+ and is used for measuring mitochondrial ATP production by mitochondrial preparations. Determination of mitochondrial ATP production is based on the different dissociation constants of Mg2+ for ADP and ATP, and the exchange of one ATP for one ADP across the mitochondrial inner membrane by the adenine nucleotide translocase (ANT). Using the dissociation constants for ADP-Mg2+ and ATP-Mg2+ and initial concentrations of ADP, ATP and Mg2+, the change in ATP concentration in the medium is calculated, which reflects mitochondrial ATP production. |
| Malate | M |
Malic acid, C4H6O5, occurs under physiological conditions as the anion malate2-, M, with pKa1 = 3.40 and pKa2 = 5.20. L-Malate is formed from fumarate in the TCA cycle in the mitochondrial matrix, where it is the substrate of malate dehydrogenase oxidized to oxaloacetate. Malate is also formed in the cytosol. It cannot permeate through the lipid bilayer of membranes and hence requires a carrier (dicarboxylate carrier, tricarboxylate carrier and 2-oxoglutarate carrier). Malate alone cannot support respiration of mt-preparations from most tissues, since oxaloacetate accumulates in the absence of pyruvate or glutamate. Malate is a type N substrate (N) required for the FAO-pathway. In the presence of anaplerotic pathways (e.g., mitochondrial malic enzyme, mtME) the capacity of the FAO-pathway can be overestimated due to a contribution of NADH-linked respiration, F(N) (see SUIT-002). |
| Malate dehydrogenase | mtMDH | Mitochondrial malate dehydrogenase is localized in the mitochondrial matrix and oxidizes malate, generated from fumarate by fumarase, to oxaloacetate, reducing NAD+ to NADH+H+ in the TCA cycle. Malate is added as a substrate in most N-pathway control states. |
| Malate-anaplerotic pathway control state | M | M: Malate alone does not support respiration of mt-preparations if oxaloacetate cannot be metabolized further in the absence of a source of acetyl-CoA. Transport of oxaloacetate across the inner mt-membrane is restricted particularly in liver. Mitochondrial citrate and 2-oxoglutarate (α-ketoglutarate) are depleted by antiport with malate. Succinate is lost from the mitochondria through the dicarboxylate carrier. OXPHOS capacity with malate alone is only 1.3% of that with Pyruvate&Malate in isolated rat skeletal muscle mitochondria. However, many mammalian and non-mammalian mitochondria have a mt-isoform of NADP+- or NAD(P)+-dependent malic enzyme (mtME), the latter being particularly active in proliferating cells. Then the anaplerotic pathway control state with malate alone (aN) supports high respiratory activities comparable to the NADH-linked pathway control states (N) with pyruvate&malate or glutamate&malate substrate combinations (PM-pathway control state, GM-pathway control state). |
| Malic enzyme | mtME | Malic enzyme (ME; EC 1.1.1.40) catalyzes the oxidative decarboxylation of L-malate to pyruvate with the concomitant reduction of the dinucleotide cofactor NAD+ or NADP+ and a requirement for divalent cations (Mg2+ or Mn2+) as cofactors.
NAD(P)+ + L-malate2- <--> NAD(P)H + pyruvate- + CO2 Three groups of ME are distinguished (i) NAD+- and (ii) NADP+-dependent ME specific for NAD+ or NADP+, respectively, and (iii) NAD(P)+- dependent ME with dual specificity for NAD+ or NADP+ as cofactor. Three isoforms of ME have been identified in mammals: cytosolic NADP+-dependent ME (cNADP-ME or ME1), mitochondrial NAD(P)+-dependent ME (mtNAD-ME or ME2; with NAD+ or NADP+ as cofactor, preference for NAD+ under physiological conditions), and mitochondrial NADP+-dependent ME (mtNADP-ME or ME3). mtNAD-ME plays an important role in anaplerosis when glucose is limiting, particularly in heart and skeletal muscle. Tartronic acid (hydroxymalonic acid) is an inhibitor of ME. |
| Malonate | Mna | Malonate (malonic acid) is a competitive inhibitor of succinate dehydrogenase (Complex II). Malonate is a substrate of malonyl-CoA synthase. |
| Mark information | Marks | » See Marks - DatLab |
| Mark statistics - DatLab | F2 | In Mark statistics one Plot is selected as a source for Marks over sections of time. Values (e.g. medians) are displayed for these time sections of the source plot and of all selected plots. |
| Matrix-ETS | matrix-ETS | The component of the electron transfer system located in the mitochondrial matrix (matrix-ETS) is distringuished from the ETS bound to the mt-inner membrane (membrane-ETS). Electron transfer and corresponding OXPHOS capacities are classically studied in mitochondrial preparations as oxygen consumption supported by various fuel substrates undergoing partial oxidation in the mt-matrix, such as pyruvate, malate, succinate, and others. |
| Melatonin | aMT | Melatonin (N-acetyl-5-methoxytryptamine, aMT) is a highly conserved molecule present in unicellular to vertebrate organisms. Melatonin is synthesized from tryptophan in the pinealocytes by the pineal gland and also is produced in other organs, tissues and fluids (extrapineal melatonin). Melatonin has lipophilic and hydrophilic nature which allows it to cross biological membranes. Therefore, melatonin is present in all subcellular compartments predominantly in the nucleus and mitochondria. Melatonin has pleiotropic functions with powerful antioxidant, anti-inflammatory and oncostatic effects with a wide spectrum of action particularly at the level of mitochondria. » MiPNet article |
| Membrane-bound ET pathway | mET-pathway | The membrane-bound electron transfer pathway (mET pathway) consists in mitochondria mainly of respiratory complexes CI, CII, electron transferring flavoprotein complex (CETF), glycerophosphate dehydrogenase complex (CGpDH), and choline dehydrogenase, with convergent electron flow at the Q-junction (Coenzyme Q), and the two downstream respiratory complexes connected by cytochrome c, CIII and CIV, with oxygen as the final electron acceptor. The mET-pathway is the terminal (downstream) module of the mitochondrial ET pathway and can be isolated from the ET-pathway in submitochondrial particles (SmtP). |
| Metabolic control variable | X | A metabolic control variable X causes the transition between a background state Y (background rate YX) and a reference state Z (reference rate ZX). X may be a stimulator or activator of flux, inducing the step change from background to reference steady state (Y to Z). Alternatively, X may be an inhibitor of flux, absent in the reference state but present in the background state (step change from Z to Y). |
| Meter | m | The meter, symbol m, is the SI unit of the SI base quantity length l. It is defined by taking the fixed numerical value of the speed of light c in vacuum to be 299 792 458 when expressed in the unit m·s−1, where the second is defined in terms of the caesium frequency ΔνCs. |
| Methylmalonic acid | Mma | Methylmalonic acid (Mma) is a common intermediate in many catabolic processes. In methylmalonic acidemia mitochondrial dysfunction can be observed, related to accumulation of Mma and associated with neurological symptoms. |
| MiP-Collection | MiP-Collection | Mitochondrial Physiology - Historical Collection
Aims The growing MiP-Collection aims at preserving scientific instruments that are of historical importance in the field of bioenergetics and mitochondrial physiology. The fast turnover of scientific equipment makes obsolete even comparatively recent instrumentation. The Oroboros O2k was the first commercial mitochondrial respirometer using a computer for data acquisition. Today, chart recorders are nearly forgotten. Due to limitations of storage space, unused scientific equipment is disposed of, despite its potential historical value. The disposal of some unique apparatus constitutes an irreversible loss to science and society, and to the continued appreciation of the foundations of our scientific discipline. You may consider to make items of scientific historical interest in mitochondrial physiology available to the MiP-Collection. These items of the MiP-Collection may specifically include historically valuable
|
| MiP03 | MiP03 | Mitochondrial Preservation Medium, MiP03, developed for preservation of isolated mitochondria. |
| MiPMap | MiPMap |
The project Mitochondrial Physiology Map (MiPMap) is initiated to provide an overview of mitochondrial properties in cell types, tissues and species. As part of Bioblast, MiPMap may be considered as an information synthase for Comparative Mitochondrial Physiology. Establishing a comprehensive database will require global input and cooperation. A comparative database of mitochondrial physiology may provide the key for understanding the functional implications of mitochondrial diversity from mouse to man, and evaluation of altered mitochondrial respiratory control patterns in health and disease (Gnaiger 2009). |
| MiPNet-Publication | MiPNet | MiPNet is the abbreviation for the OROBOROS Journal Mitochondrial Physiology Network, including chapters of the O2k-Manual, O2k-Procedures, O2k-Workshops, and other announcements, starting with MiPNet 01 in 1996. See also »MiPNet. |
| MiPSociety | MiP | The Mitochondrial Physiology Society (MiP) has been founded to organize MiPconferences, MiPschools, and MiPworkshops worldwide. MiP has been founded at the Third Conference on Mitochondrial Physiology (MiP2003, Schroecken, Austria). The MiPsociety is an international organization, based in Europe and operating world-wide. |
| MiR05 | MiR05 | Mitochondrial respiration medium, MiR05, developed for oxygraph incubations of mitochondrial preparations. Respiration of living cells may be assessed in MiR05 by adding pyruvate (P) as an external source. MiR06 = MiR05 + catalase. MiR05Cr = MiR05 + creatine. |
| MiR05Cr | MiR05Cr | Mitochondrial respiration medium, MiR05Cr, developed for oxygraph incubations of mitochondrial preparations - permeabilized muscle fibers. MiR05Cr = MiR05 + 20 mM creatine. |
| MiR06 | MiR06 | Mitochondrial respiration medium, MiR06, developed for oxygraph incubations of mitochondrial preparations. MiR06 = MiR05 plus catalase. MiR06Cr = MiR06 plus creatine. |
| MiR06Cr | MiR06Cr | Mitochondrial respiration medium, MiR06Cr, developed for oxygraph incubations of mitochondrial preparations - permeabilized muscle fibers. MiR06Cr = MiR06 + 20 mM creatine. |
| MiRK03 | MiRK03 | Mitochondrial respiration medium, MiRK03, modified after a medium described by Komary 2010 Biochim Biophys Acta, intended for use as medium for H2O2 production measurement with Amplex Red. |
| Microxia | microx | Microxia (deep hypoxia) is obtained when trace amounts of O2 exert a stimulatory effect on respiration above the level where metabolism is switched to a purely anaerobic mode. |
| MitoAction | MitoAction |  The mission of MitoAction is to improve quality of life for all who are affected by mitochondrial disorders through support, education and advocacy initiatives. The mission of MitoAction is to improve quality of life for all who are affected by mitochondrial disorders through support, education and advocacy initiatives. |
| MitoCanada Foundation | mitoCanada | The MitoCanada Foundation.
The MitoCanada Foundation is Canada’s only not-for-profit organization focused on mitochondrial disease. Since its founding in 2010, MitoCanada has dedicated over $1 million to fund the work of leading Canadian scientists and to support national awareness and support programs. The MitoCanada Foundation is committed to ensuring that those who live with mitochondrial disease are able to enjoy the best possible quality of life until there is a cure. |
| MitoFit DOI Data Center | MitoFit DOI DC | The MitoFit DOI Data Center is responsible for the provision of digital identifiers, for the storage and ensuring the persistence of the scientific objects, the provision of access, review process and maintenance of the Metadata, and quality control. |
| MitoFit Preprints | MitoFit Prep | MitoFit Preprints is an Open Access preprint server for mitochondrial physiology and bioenergetics. |
| MitoFit registered project | MitoFit-RP | MitoFit registered projects are announced with reference to MitoFit protocols as publicly deposited protocols. Project registration is a two-phase process. Guidelines will be defined. (1) Pre-registration of a project requires submission to a MitoFit moderator (editor), including protocol details with reference to MitoPedia protocols, or with submission of protocols for publication (Open Access) in MitoPedia. The MitoFit (Bioblast) editors will edit the submitted protocols (layout) and insert into Bioblast submitted pre-registrations and protocols. (2) MitoFit moderators (editors) will set up a MitoFit accreditation panel, in which the registrant will be included (perhaps not in the long run, to avoid conflict of interests) and/or for which the registrant can suggest delegates (compare peer review). Accredited MitoFit protocols are labelled as MitoFit accredited, and the pre-registered MitoFit project becomes labelled and listed as MitoFit registered project (MitoFit accredited). This is possible before (advance registration), during progress, and after completion of a study (post-registration). A MitoFit registered project receives a code for feeding data into the MitoFit data repository. |
| MitoKit-CII/Malonate-nv | Mnanv | MitoKit-CII/Malonate-nv (diacetoxymethyl malonate) is a plasma membrane-permeable prodrug (permeable malonate; Mnanv) that diffuses across the plasma membrane. Cleavage of diacetoxymethyl groups is mediated by intracellular esterases, thus releasing malonate in the intracellular space. Abliva #: 01-161-s2 |
| MitoKit-CII/Succinate-nv | Snv | MitoKit-CII/Succinate-nv (diacetoxymethyl succinate) is a plasma membrane-permeable prodrug (permeable succinate; Snv) that diffuses across the plasma membrane. Cleavage of diacetoxymethyl groups is mediated by intracellular esterases, thus releasing succinate in the intracellular space. Abliva #: 01-118-s4 |
| MitoOx1 | MitoOx1 | Mitochondrial respiration medium, MitoOx1, used by the Budapest groups for respirometry und Amplex Red trials. |
| MitoOx2 | MitoOx2 | Mitochondrial respiration medium, MitoOx2, developed for oxygraph incubations of mitochondrial preparations to measure the H2O2 production. MitoOx2 yields a higher optical sensitivity and lower "drift" (oxidation of the fluorophore precurcor without H2O2 present) for Amplex UltraRed(R) than e.g. MiR05. |
| Mitochondria | mt | Mitochondria (Greek mitos: thread; chondros: granule) are small structures within cells, which function in cell respiration as powerhouses or batteries. Mitochondria belong to the bioblasts of Richard Altmann. Abbreviation: mt, as generally used in mtDNA. Singular: mitochondrion (bioblast); plural: mitochondria (bioblasts). |
| Mitochondria Interest Group | MIG | The Mitochondria Interest Group (MIG) is an Inter-Institute Interest Group at the National Institutes of Health (NIH), with members worldwide! MIG is concerned with all aspects of the mitochondrion and diseases in which the mitochondrion is involved. We hold monthly meetings, usually on the second Monday of the month (except when it is a Federal holiday or other special exceptions). MITOCHONDRIA-L@LIST.NIH.GOV is an Email list moderated by Ph.D. Steven Zullo as an interactive information platform, with free subscritpion to this mitochondrial network. List members are reminded of their responsibility to critically evaluate the content of the postings. The information, opinions, data, and statements contained herein are not necessarily those of the U. S. Government, the National Institutes of Health (NIH), or MIG and should not be interpreted, acted on or represented as such. |
| Mitochondria Research Society | MRS | The Mitochondria Research Society (MRS) is a nonprofit international organization of scientists and physicians. The purpose of MRS is to find a cure for mitochondrial diseases by promoting research on basic science of mitochondria, mitochondrial pathogenesis, prevention, diagnosis and treatment through out the world. |
| Mitochondria-Targeted Drug Development | hansonwade |
The Mitochondria-Targeted Drug Development Summit was first established in 2021, as an online conference. Due to its success and unmatched focus, the 2nd edition returns to Boston this March 2022. This is the only industry-led meeting that unites key stakeholders under a mutual and ambitious objective of accelerating the discovery and development of novel drugs that target mitochondrial functions for chronic, primary mitochondrial diseases, muscular dystrophy, metabolic disorders, and neurodegenerative diseases. Join our speakers from GenSight Biologics, Abliva, Reneo Pharma, Mito BioPharma, Mitokinin and more with exciting networking opportunities, panel discussions and dedicated roundtables. |
| Mitochondrial ATP-sensitive K+ channel | mtKATP | The mitochondrial ATP-sensitive K+ channel (mtKATP or mitoKATP). |
| Mitochondrial European Education Training | MEET | The Mitochondrial European Education Training (MEET) MEET is a project started on January 2013. MEET network is composed by a multi-partner project that intends to mobilize the critical mass of expertise, by linking partners from 8 different countries, among which 8 world-leading basic science and clinical centers of excellence, an 1 SME with direct interest in mitochondrial medicine and 3 associated partners that provide for all trainees no-scientific training. MEET is training 11 ESRs and 3 ERs coming from all over the world supervised in their research by 15 mentors and by their collaborators. MEET combine the efforts of leading clinicians with those of more basic oriented groups and will have important implications for the comprehension and treatment of mitochondria-related pathologies. |
| Mitochondrial Medicine Society | MMS | The Mitochondrial Medicine Society (MMS) was founded in 2000 and represents an international group of physicians, researchers and clinicians working towards the better diagnosis, management, and treatment of mitochondrial diseases. |
| Mitochondrial Physiology Network | Mitochondr physiol network, MiPNet | The Mitochondrial Physiology Network is the on-line Oroboros journal. |
| Mitochondrial competence | mt-competence; MitoCom | Mitochondrial metabolic competence is the organelle's capacity to provide adequate amounts of ATP in due time, by adjusting the mt-membrane potential, mt-redox states and the ATP/ADP ratio according to the metabolic requirements of the cell.
The term mitochondrial competence is also known in a genetic context: Mammalian mitochondria possess a natural competence for DNA import. MitoCom_O2k-Fluorometer is a Mitochondrial Competence network, the nucleus of which is formed by the K-Regio project MitoCom Tyrol. |
| Mitochondrial concentration | CmtE | Mitochondrial concentration is CmtE = mtE·V-1 [mtEU·m-3]. mt-Concentration is an experimental variable, dependent on sample concentration. |
| Mitochondrial content | mtENX | Mitochondrial content per object X is mtENX = mtE·NX-1 [mtEU·x-1]. |
| Mitochondrial density | DmtE | Specific mitochondrial density is DmtE = mtE·mX-1 [mtEU·kg-1]. If the amount of mitochondria, mtE, is expressed as mitochondrial mass, then DmtE is the mass fraction of mitochondria in the sample. If mtE is expressed as mitochondrial volume, Vmt, and the mass of sample, mX, is replaced by volume of sample, VX, then DmtE is the volume fraction of mitochondria in the sample. |
| Mitochondrial free radical theory of aging | MFRTA | The mitochondrial free radical theory of aging goes back to Harman (1956) and ranks among the most popular theories of aging. It is based on postulates which are not unequivocally supported by observation (Bratic, Larsson 2013):
(i) Mitochondrial ROS production increases with age caused by progressive mitochondrial dysfunction; (ii) antioxidat capacity declines with age; (iii) mutations of somatic mtDNA accumulate during aging; (iv) a vicious cycle occurs of increased ROS production caused by mtDNA mutations and degenerated mt-function, and due to ROS-induced ROS production. |
| Mitochondrial inner membrane | mtIM | The mitochondrial inner membrane mtIM is the structure harboring the membrane-bound electron transfer system ETS including the respiratory complexes working as hydrogen ion pumps, the mt-phosphorylation system including the hydrogen ion pump ATP synthase, several substrate transporters involved in the electron transfer pathway, and a variety of other ion pumps that carry proton charge (Ca2+, Mg2+). The protonmotive force is the electrochemical potential difference across the mtIM generated by the hydrogen ion pumps of the . |
| Mitochondrial marker | mt-marker | Mitochondrial markers are structural or functional properties that are specific for mitochondria. A structural mt-marker is the area of the inner mt-membrane or mt-volume determined stereologically, which has its limitations due to different states of swelling. If mt-area is determined by electron microscopy, the statistical challenge has to be met to convert area into a volume. When fluorescent dyes are used as mt-marker, distinction is necessary between mt-membrane potential dependent and independent dyes. mtDNA or cardiolipin content may be considered as a mt-marker. Mitochondrial marker enzymes may be determined as molecular (amount of protein) or functional properties (enzyme activities). Respiratory capacity in a defined respiratory state of a mt-preparation can be considered as a functional mt-marker, in which case respiration in other respiratory states is expressed as flux control ratios. » MiPNet article |
| Mitochondrial matrix | mt-matrix | The mitochondrial matrix (mt-matrix) is enclosed by the mt-inner membrane mtIM. The terms mitochondrial matrix space or mitochondrial lumen are used synonymously. The mt-matrix contains the enzymes of the tricarboxylic acid cycle, fatty acid oxidation and a variety of enzymes that have cytosolic counterparts (e.g. glutamate dehydrogenase, malic enzyme). Metabolite concentrations, such as the concentrations of fuel substrates, adenylates (ATP, ADP, AMP) and redox systems (NADH), can be very different in the mt-matrix, the mt-intermembrane space, and the cytosol. The finestructure of the gel-like mt-matrix is subject of current research. |
| Mitochondrial membrane potential | mtMP, ΔΨp+, ΔelFep+ [V] | The mitochondrial membrane potential difference, mtMP or ΔΨp+ = ΔelFep+, is the electric part of the protonmotive force, Δp = ΔmFeH+.
|
| Mitochondrial outer membrane | mtOM | The mitochondrial outer membrane is the incapsulating membrane which is osmotically not active and contains the cytochrome b5 enzyme similar to that found in the endoplasmatic reticulum, the translocases of the outer membrane, monoaminooxidase, the palmitoyl-CoA synthetase and carnytil-CoA transferase 1. |
| Mitochondrial preparations | mtprep | Mitochondrial preparations (mtprep) are isolated mitochondria (imt), tissue homogenate (thom), mechanically or chemically permeabilized tissue (permeabilized fibers, pfi) or permeabilized cells (pce). In mtprep the plasma membranes are either removed (imt) or mechanically (thom) and chemically permeabilized (pfi), while mitochondrial functional integrity and to a large extent mt-structure are maintained in incubation media optimized to support mitochondrial physiological performance. According to this definition, submitochondrial particles (smtp) are not a mtprep, since mitochondrial structure is altered although specific mitochondrial functions are preserved. |
| Mitochondrial respiration media: comparison | MiR | Mitochondrial respiratory capacity and control are compared in different mitochondrial respiration media, MiRs, to evaluate the quality of MiRs in preserving mitochondrial function and to harmonize results obtained in various studies using different MiRs. In some cases alterations of the formulation are incorporated to optimize conditions for the simultaneous measurement of multiple parameters, e.g. respiration and ROS production. |
| Mitochondrial states and rates - terminology beyond MitoEAGLE 2020 | States and rates | 666 coauthors of the 'MitoEAGLE white paper' [1] collaborated to reach a consensus on terminology related to mitochondrial respiratory states and rates. This page is intended to prepare a questionnaire and follow-up publication. |
| Mitochondrial transcription factor A | TFAM | The transcription factor A is a gene that encodes a mitochondrial transcription factor that is a key activator of mitochondrial transcription as well as a participant in mitochondrial genome replication. TFAM is downstream of PGC-1alpha. |
| Molar mass | M [kg·mol-1]; [g·mol-1] | Molar mass M is the mass of a chemical compound divided by its amount-of-substance measured in moles. It is defined as MB = m/nB, where m is the total mass of a sample of pure substance and nB is the amount of substance B given in moles. The definition applies to pure substance. The molar mass allows for converting between the mass of a substance and its amount for bulk quantities. It is calculated as the sum of standard atomic weights of all atoms that form one entity of the substance. The appropriate SI base units is kg·mol-1. However, for historical as well as usability reasons, g·mol-1 is almost always used instead. |
| Mole | mol | The mole [mol] is the SI base unit for the amount of substance of a system that contains 6.02214076·1023 specified elementary entities (see Avogadro constant). The elementary entities must be specified and may be atoms, molecules, ions, electrons, other particles, or specified groups of such particles. |
| Monoamine oxidase | MAO | Monoamine oxidases are enzymes bound to the outer membrane of mitochondria and they catalyze the oxidative deamination of monoamines. Oxygen is used to remove an amine group from a molecule, resulting in the corresponding aldehyde and ammonia. Monoamine oxidases contain the covalently bound cofactor FAD and are, thus, classified as flavoproteins. |
| Motive entity | Xtr [MU] | . A motive entity Xtr is an entity involved in a transformation including spacial transfer. Motive entities (transformants) are expressed in different motive units [MU] depending on the energy transformation under study and the chosen format. Flows are defined as advancement in terms of stoichiometric motive entities per time. Isomorphic forces are partial derivatives of Gibbs energy per advancement. Ions carrying a positive charge (cations) or negative charge (anions) may be considered as a paradigm of motive entities, since Faraday did not coin but introduced the term 'ion', which is old Greek for 'going' — advancing to the cathode or anode and thus generating an electric current. |
| Motive unit | MU | The motive unit [MU] is the variable SI unit in which the motive entity (transformant) of a transformation is expressed, which depends on the energy transformation under study and on the chosen format. Fundamental MU for electrochemical transformations are:
For the protonmotive force the motive entity is the proton with charge number z=1. The protonmotive force is expressed in the electrical or molar format with MU J/C=V or J/mol=Jol, respectively. The conjugated flows, I, are expressed in corresponding electrical or molar formats, C/s = A or mol/s, respectively. The charge number, z, has to be considered in the conversion of motive units (compare Table below), if a change not only of units but a transition between the entity elementary charge and an entity with charge number different from unity is involved (e.g., O2 with z=4 in a redox reaction). The ratio of elementary charges per reacting O2 molecule (zO2=4) is multiplied by the elementary charge (e, coulombs per proton), which yields coulombs per O2 [C∙x-1]. This in turn is multiplied with the Avogadro constant, NA (O2 molecules per mole O2 [x∙mol-1]), thus obtaining for zeNA the ratio of elementary charges [C] per amount of O2 [mol-1]. The conversion factor for O2 is 385.94132 C∙mmol-1. |
| Mouse control: Mark | Ctrl+M | The mark mode is active by default, can be selected in the menu or by [Ctrl+M]. If Mouse control: Mark is enabled, specific sections of the experiment can be marked in each plot.
Usually, marks are set on the plot for oxygen concentration for calibration, whereas marks on the plot for oxygen flux are set for exporting the median or average of flux to a table. »More details: Marks - DatLab. |
| Mouse control: Zoom | Ctrl+Z | Select Mouse Control: Zoom in the Graph-menu or press [Ctrl+Z]. |
| MtOM | mtOM | The mitochondrial outer membrane |
| Myxothiazol | Myx | Myxothiazol Myx is an inhibitor of Complex III (CIII). CIII also inhibits CI. Myxothiazol binds to the Qo site of CIII (close to cytochrome bL) and inhibits the transfer of electrons from reduced QH2 to the Rieske iron sulfur protein. |
| N-ethylmaleimide | Nem | N-ethylmaleimide is an organic compound that is derived from maleic acid and blocks endogenous Pi transport. |
| N/NS pathway control ratio | N/NS | The N/NS pathway control ratio is obtained when succinate is added to N-linked respiration in a defined coupling state. N and NS are abbreviations for respiration in the N-pathway control state (with pyruvate, glutamate, malate, or other ETS competent N-linked substrate combinations) and the NS-pathway control state (N in combination with succinate). NS indicates respiration with a cocktail of substrates supporting the N- and S-pathways. |
| N/S pathway control ratio | N/S | The N/S pathway control ratio is obtained from SUIT protocols when the N-pathway flux and S-pathway flux are measured in the same coupling control state. The N/S pathway control ratio may be larger or smaller than 1.0, depending on the mitochondrial source and various mitochondrial injuries. The S-pathway control state may be selected preferentially as reference state, if mitochondria are studied with respect to N-pathway injuries. |
| NADH | NADH | NAD+ and NADH: see Nicotinamide adenine dinucleotide. |
| NADH electron transfer-pathway state | N | The NADH electron transfer-pathway state (N) is obtained by addition of NADH-linked substrates (CI-linked), feeding electrons into the N-junction catalyzed by various mt-dehydrogenases. N-supported flux is induced in mt-preparations by the addition of NADH-generating substrate combinations of pyruvate (P), glutamate (G), malate (M), oxaloacetate (Oa), oxoglutarate (Og), citrate, hydroxybutyrate. These N-junction substrates are (indirectly) linked to Complex I by the corresponding dehydrogenase-catalyzed reactions reducing NAD+ to NADH+H+ + H+. The most commonly applied N-junction substrate combinations are: PM, GM, PGM. The malate-anaplerotic pathway control state (M alone) is a special case related to malic enzyme (mtME). The glutamate-anaplerotic pathway control state (G alone) supports respiration through glutamate dehydrogenase (mtGDH). Oxidation of tetrahydrofolate is a NAD(P)H linked pathway with formation of formate. In mt-preparations, succinate dehydrogenase (SDH; CII) is largely substrate-limited in N-linked respiration, due to metabolite depletion into the incubation medium. The residual involvement of S-linked respiration in the N-pathway control state can be further suppressed by the CII-inhibitor malonic acid). In the N-pathway control state ET pathway level 4 is active. |
| NS e-input | NS, CI&II | NS e-input or the NS-pathway control state is electron input from a combination of substrates for the N-pathway control state and S-pathway control state through Complexes CI and CII simultaneously into the Q-junction. NS e-input corresponds to TCA cycle function in vivo, with convergent electron flow through the Electron transfer pathway. In mt-preparations, NS e-input requires addition not only of NADH- (N-) linked substrates (pyruvate&malate or glutamate&malate), but of succinate (S) simultaneously, since metabolite depletion in the absence of succinate prevents a significant stimulation of S-linked respiration. For more details, see: Additive effect of convergent electron flow. |
| NS-N pathway control efficiency | jNS-N; jCI&II-CI | The NS-N pathway control efficiency, jNS-N = 1-N/NS, expresses the fractional change of flux when succinate is added to the N-pathway control state in a defined coupling-control state. |
| NS-S pathway control efficiency | jNS-S | The NS-S pathway control efficiency expresses the relative stimulation of succinate supported respiration (S) by NADH-linked substrates (N), with the S-pathway control state as the background state and the NS-pathway control state as the reference state. In typical SUIT protocols with type N and S substrates, flux in the NS-pathway control state NS is inhibited by rotenone to measure flux in the S-pathway control state, S(Rot) or S. Then the NS-S pathway control efficiency in the ET-coupling state is
j(NS-S)E = (NSE-SE)/NSEThe NS-S pathway control efficiency expresses the fractional change of flux in a defined coupling-control state when inhibition by rotenone is removed from flux under S-pathway control in the presence of a type N substrate combination. Experimentally rotenone Rot is added to the NS-state. The reversed protocol, adding N-substrates to a S-pathway control background does not provide a valid estimation of S-respiration with succinate in the absence of Rot, since oxaloacetate accumulates as a potent inhibitor of succinate dehydrogenase CII. |
| NS-pathway control state | NS, CI&II | NS-pathway control is exerted in the NS-linked substrate state (flux in the NS-linked substrate state, NS; or Complex I&II, CI&II-linked substrate state). NS-OXPHOS capacity provides an estimate of physiologically relevant maximum mitochondrial respiratory capacity. NS is induced in mt-preparations by addition of NADH-generating substrates (N-pathway control state in combination with succinate (Succinate pathway; S). Whereas NS expresses substrate control in terms of substrate types (N and S), CI&II defines the same concept in terms of convergent electron transfer to the Q-junction (pathway control). NS is the abbreviation for the combination of NADH-linked substrates (N) and succinate (S). This physiological substrate combination is required for partial reconstitution of TCA cycle function and convergent electron-input into the Q-junction, to compensate for metabolite depletion into the incubation medium. NS in combination exerts an additive effect of convergent electron flow in most types of mitochondria. |
| NSGp | NSGp |
MitoPathway control state: NSGp
SUIT protocol: SUIT-038 This substrate combination supports convergent electron flow to the Q-junction. |
| Natoms O | natoms O | 0.5 nmol O2; in bioenergetics a variety of expressions is used for units of amount of half a nmol molecular oxygen (natoms oxygen; natoms O; ng.atom O; nmol O), with the identical meaning: 0.5 nmol O2. |
| Net P/E control ratio | (P-L)/E | |
| Net R/E control ratio | (R-L)/E | |
| Neurocon | Neurocon | Neurocon is an Indian society organizing international conferences on neurodegenerative and neurodevelopmental diseases. |
| Nicotinamide adenine dinucleotide | NADH | Nicotinamide adenine dinucleotide, NAD+ and NADH (pyridine nucleotide coenzymes, NAD and NADP), is an oxidation-reduction coenzyme (redox cofactor; compare FADH2). In the NADH electron transfer-pathway state fuelled by type N-substrates, mt-matrix dehydrogenases generate NADH, the substrate of Complex I (CI). The reduced N-substrate RH2 is oxidized and NAD+ is reduced to NADH,:::: RH2 + NAD+ → NADH + H+ + RThe mt-NADH pool integrates the activity of the TCA cycle and various matrix dehydrogenases upstream of CI, and thus forms a junction or funnel of electron transfer to CI, the N-junction (compare F-junction, Q-junction). NAD+ and NADH are not permeable through the mt-inner membrane, mtIM. Therefore, an increase of mitochondrial respiration after the addition of NADH may indicate an alteration of the mtIM integrity. Cytosolic NADH is effectively made available for mitochondrial respiration through the malate-aspartate shuttle or glycerophosphate dehydrogenase Complex. |
| Nitric oxide synthase | NOS | Nitric oxide synthase, NOS, catalyzes the production of nitric oxide (NO•), which is a reactive nitrogen species. There are four types of NOS: neuronal NOS (nNOS), endothelial NOS (eNOS), inducible NOS (iNOS) and mitochondrial NOS (mtNOS). |
| Noncoupled respiration | E | Noncoupled respiration is maximum electron flow in an open-transmembrane proton circuit mode of operation (see ET capacity). » MiPNet article |
| Normoxia | normox | Normoxia is a reference state, frequently considered as air-level oxygen pressure at sea level (c. 20 kPa in water vapor saturated air) as environmental normoxia. Intracellular tissue normoxia is variable between organisms and tissues, and intracellular oxygen pressure is frequently well below air-level pO2 as a result of cellular (mainly mitochondrial) oxygen consumption and oxygen gradients along the respiratory cascade. Oxygen pressure drops from ambient normoxia of 20 kPa to alveolar normoxia of 13 kPa, while extracellular normoxia may be as low as 1 to 5 kPa in solid organs such as heart, brain, kidney and liver. Pericellular pO2 of cells growing in monolayer cell cultures may be hypoxic compared to tissue normoxia when grown in ambient normoxia (95 % air and 5 % CO2) and a high layer of culture medium causing oxygen diffusion limitation at high respiratory activity, but pericellular pO2 may be effectively hyperoxic in cells with low respiratory rate with a thin layer of culture medium (<2 mm). Intracellular oxygen levels in well-stirred suspended small cells (5 - 7 mm diameter; endothelial cells, fibroblasts) are close to ambient pO2 of the incubation medium, such that matching the experimental intracellular pO2 to the level of intracellular tissue normoxia requires lowering the ambient pO2 of the medium to avoid hyperoxia. |
| Nuclear receptors | NRs | Nuclear receptors are ligand-dependent transcription factors. |
| Nuclear respiratory factor 1 | NRF-1 | Nuclear respiratory factor 1 is a transcription factor downstream of PGC-1alpha involved in coordinated expression of nDNA and mtDNA. |
| Number | N | A number N is a count NX [x] divided by the elementary entity UX [x]. X must represent the same entity in both occurences. The elementary unit [x] cancels in the division by simplification, such that numbers (for example, numbers 8 or 24) are abstracted from the counted entity X. The concept of number is tightly entangled with units, counts and entities. |
| O2k | O2k | O2k - Oroboros O2k: the modular system for high-resolution respirometry. |
| O2k control | F7 | After selection of an O2k setup in the O2k control [F7] window, followed by a left-click Send to O2k, only the following control functions are routinely required during experimental operations. |
| O2k-Network Reference Laboratory | O2k-Network Lab | O2k-Network Reference Laboratories build a WorldWide network on high-resolution respirometry and mitochondrial physiology, the Oroboros O2k-Network. |
| O2k-sV-Module | O2k-sV-Module | The O2k-sV-Module is the O2k small-volume module, comprised of two Duran® glass chambers of 12 mm inner diameter specifically developed to perform high-resolution respirometry with reduced amounts of biological sample, and all the components necessary for a smaller operation volume V of 0.5 mL. The current DatLab version is included in the delivery of this revolutionary module. |
| OXPHOS capacity | P | |
| OctGM | OctGM | OctGM: Octanoylcarnitine & Glutamate & Malate.
MitoPathway control state: FN SUIT protocols: SUIT-015, SUIT-016, SUIT-017 |
| OctGMS | OctGMS | OctGMS: Octanoylcarnitine &Glutamate & Malate& Succinate.
MitoPathway control state: FNS SUIT protocols: SUIT-016, SUIT-017 |
| OctM pathway control state | OctM | OctM: Octanoylcarnitine & Malate.
MitoPathway control state: F SUIT protocols: SUIT-002, SUIT-015, SUIT-016, SUIT-017 Respiratory stimulation of the FAO-pathway, F, by fatty acid FA in the presence of malate M. Malate is a type N substrate (N), required for the F-pathway. In the presence of anaplerotic pathways (e.g., mitochondrial malic enzyme, mtME) the F-pathway capacity is overestimated, if there is an added contribution of NADH-linked respiration, F(N) (see SUIT-002). The FA concentration has to be optimized to saturate the FAO-pathway, without inhibiting or uncoupling respiration. Low concentration of malate, typically 0.1 mM, does not saturate the N-pathway; but saturates the F-pathway. High concentration of malate, typically 2 mM, saturates the N-pathway. |
| OctPGM pathway control state | OctPGM | OctPGM: Octanoylcarnitine & Pyruvate & Glutamate & Malate.
MitoPathway control state: FN SUIT protocols: SUIT-002
|
| OctPGMS pathway control state | OctPGMS | OctPGMS: Octanoylcarnitine & Pyruvate & Glutamate & Malate & Succinate.
MitoPathway control state: FNS SUIT protocol: SUIT-001, SUIT-002, SUIT-015 This substrate combination supports convergent electron flow to the Q-junction. |
| OctPGMSGp pathway control state | OctPGMSGp | OctPGMSGp: Octanoylcarnitine & Pyruvate & Glutamate & Malate & Succinate & Glycerophosphate.
MitoPathway control state: FNSGp SUIT protocol: SUIT-002 This substrate combination supports convergent electron flow to the Q-junction. |
| OctPM pathway control state | OctPM | OctPM: Octanoylcarnitine & Pyruvate & Malate.
MitoPathway control state: FN SUIT protocol: SUIT-002, SUIT-005 This substrate combination supports N-linked flux which is typically higher than FAO capacity (F/FN<0 in the OXPHOS state). In SUIT-RP1, PMOct is induced after PM(E), to evaluate any additive effect of adding Oct. In SUIT-RP2, FAO OXPHOS capacity is measured first, testing for the effect of increasing malate concentration (compare malate-anaplerotic pathway control state, M alone), and pyruvate is added to compare FAO as the background state with FN as the reference state. |
| OctPMS | OctPMS | OctPMS: Octanoylcarnitine & Pyruvate & Malate & Succinate.
MitoPathway control state: FNS SUIT protocol: SUIT-005 |
| Octanoate | Oca | Octanoate (octanoic acid). C8H16O2 Common name: Caprylic acid. |
| Octanoylcarnitine | Oct | Octanoylcarnitine is a medium-chain fatty acid (octanoic acid: eight-carbon saturated fatty acid) covalently linked to carnitine, frequently applied as a substrate for fatty acid oxidation (FAO) in mitochondrial preparations. |
| Oligomycin | Omy | Oligomycin (Omy) is an inhibitor of ATP synthase by blocking its proton channel (Fo subunit), which is necessary for oxidative phosphorylation of ADP to ATP (energy production). The inhibition of ATP synthesis also inhibits respiration. In OXPHOS analysis, Omy is used to induce a LEAK respiration state of respiration (abbreviated as L(Omy) to differentiate from L(n), LEAK state in the absence of ADP). |
| Open - DatLab | Ctrl+O | Open a previously recorded DatLab file. |
| Open Access | OA | Open Access (OA) academic articles comprise all different forms of published research that are distributed online, free of charge and with an open license to facilitate the distribution and reuse. The open access repositories serve as the perfect vehicle to transmit free knowledge, including but not limited to peer-reviewed and non-peer-reviewed academic journal articles, conference papers, theses, book chapters and monographs. Driven by the problems of social inequality caused by restricting access to academic research, the Open Access movement changes the funding system of published literature allowing for more readers and thus increased access to scientific knowledge, as well as addressing the economic challenges and unsustainability of academic publishing. In addition to being free to read (gratis), open access articles may also be free to use (libre) where the copyright is held by the authors and not the publisher. Definition by the Directory of Open Access Journals (DOAJ): "We define these as journals where the copyright holder of a scholarly work grants usage rights to others using an open license (Creative Commons or equivalent) allowing for immediate free access to the work and permitting any user to read, download, copy, distribute, print, search, or link to the full texts of articles, crawl them for indexing, pass them as data to software, or use them for any other lawful purpose." |
| Open Science | OS | Building on the essential principles of academic freedom, research integrity and scientific excellence, open science sets a new paradigm that integrates into the scientific enterprise practices for reproducibility, transparency, sharing and collaboration resulting from the increased opening of scientific contents, tools and processes. Open science is defined as an inclusive construct that combines various movements and practices aiming to make multilingual scientific knowledge openly available, accessible and reusable for everyone, to increase scientific collaborations and sharing of information for the benefits of science and society, and to open the processes of scientific knowledge creation, evaluation and communication to societal actors beyond the traditional scientific community. It comprises all scientific disciplines and aspects of scholarly practices, including basic and applied sciences, natural and social sciences and the humanities, and it builds on the following key pillars: open scientific knowledge, open science infrastructures, science communication, open engagement of societal actors and open dialogue with other knowledge systems. |
| Open chamber | O | The term "open O2k-chamber" refers to a situation in which the liquid phase is allowed to equilibrate with a gas phase, but the stopper is partially inserted using the Stopper-Spacer. |
| Ordinate | y | The ordinate is the vertical axis y of a rectangular two-dimensional graph with the abscissa x as the horizontal axis. Values Y are placed vertically from the origin. See Ordinary Y/X regression. |
| Ouabain | Oua | Ouabain (synonym: G-strophantin octahydrate) is a poisonous cardiac glycoside. The classical mechanism of action of ouabain involves its binding to and inhibition of the plasma membrane Na+/K+-ATPase (sodium pump) especially at the higher concentrations. Low (nanomolar and subnanomolar) concentrations of ouabain stimulate the Na-K-ATPase. |
| Outlier-skewness index | OSI, OI | An outlier-skewness index OSI is defined for evaluation of the distribution of data sets with outliers including separate clusters or skewness in relation to a normal distribution with equivalence of the average and median. The OSI is derived from Pearson’s coefficient of skewness 2:
The outlier-skewness index OSI introduces the absolute value of the arithmetic mean, m = ABS(average + median)/2, for normalization:
|
| Oxaloacetate | Oa | Oxaloacetic acid, C4H4O5, occurs under physiological conditions as the anion oxaloacetate2-, Oa. Oxaloacetate is formed from malate by MDH. Oa reacts with acetyl-CoA through citrate synthase to form citrate, or with glutamate through transaminase to form oxoglutarate and aspartate. Oa transport is restricted across the inner mt-membrane of various tissues. Oa is a potent inhibitor of succinate dehydrogenase. |
| Oxidative phosphorylation | OXPHOS | |
| Oxoglutarate | Og | 2-Oxoglutaric acid or alpha-ketoglutaric acid, C5H6O5, occurs under physiological conditions as the anion 2-Oxoglutarate2-, Og. 2-Oxoglutarate (alpha-ketoglutarate) is formed from isocitrate as a product of isocitrate dehydrogenase (IDH) in the TCA cycle, and is a substrate of oxoglutarate dehydrogenase (OgDH). The 2-oxoglutarate carrier exchanges malate2- for 2-oxoglutarate2- as part of the malate-aspartate shuttle. In the cytosol, oxoglutarate+aspartate are transaminated to form oxaloacetate+glutamate. Cytosolic malate dehydrogenase converts oxaloacetate+NADH to malate. |
| Oxoglutarate dehydrogenase | OgDH | Oxoglutarate dehydrogenase (α-ketoglutarate dehydrogenase) is a highly regulated enzyme of the tricarboxylic acid cycle. It catalyses the conversion of oxoglutarate (alpha-ketoglutarate) to succinyl-CoA, reduces NAD+ to NADH and thus links to Complex I in the Electron transfer-pathway. OgDH is activated by low Ca2+ (<20 µM) but inactivated by high Ca2+ (>100 µM). OgDH is an important source of ROS. |
| Oxycaloric equivalent | DeltakHO2 | The oxycaloric equivalent is the theoretically derived enthalpy change of the oxidative catabolic reactions per amount of oxygen respired, DeltakHO2, ranging from -430 to -480 kJ/mol O2. The oxycaloric equivalent is used in indirect calorimetry to calculate the theoretically expected metabolic heat flux from the respirometrically measured metabolic oxygen flux. Calorimetric/respirometric ratios (CR ratios; heat/oxygen flux ratios) are experimentally determined by calorespirometry. A CR ratio more exothermic than the oxycaloric equivalent of -480 kJ/mol indicates the simultaneous involvement of aerobic and anaerobic mechanisms of energy metabolism. |
| Oxygen | O2 | Molecular oxygen, O2 or dioxygen, has two atoms of oxygen, O, which is the chemical element with atomic number 8. The relative molecular mass of O2, Mr,O2, is 32 (or 31.9988). The element O has 8 protons, 8 neutrons and 8 electrons. In the figure, the two electrons in the first electron shell are not shown. Of the six electrons in the outer shell (blue bullets), one electron from each of the two atoms is shared in O2 forming the covalent bond, and one electron in each atom is unpaired. |
| Oxygen flow | IO2 [mol·s-1] or [mol·s-1·x-1] | Respiratory oxygen flow is the oxygen consumption per total system, which is an extensive quantity. Flow is advancement of a transformation in a system per time [mol·s-1], when 'system' is defined as the experimental system (e.g. an open or closed chamber). Flow is distinguished from the size-specific quantity flux obtained by normalization of flow per volume of the experimental system [mol·s-1·m-3]. An experimental object, e.g. a living cell, may be considered as the 'experimental system'. Then oxygen flow per cell has the unit [mol·s-1·x-1], where [x] is the elementary unit for a count. Oxygen flow or respiration per cell [amol·s-1·x-1] = [pmol·s-1·Mx-1] is normalized for the cell count, distinguished from oxygen flux (e.g. per mg protein or wet mass). These are different forms of normalization of rate. |
| Oxygen flux | JO2 | Oxygen flux, JO2, is a specific quantity. Oxygen flux is oxygen flow, IO2 [mol·s-1 per system] (an extensive quantity), divided by system size. Flux may be volume-specific (flow per volume [pmol·s-1·mL-1]), mass-specific (flow per mass [pmol·s-1·mg-1]), or marker-specific (flow per mtEU). Oxygen flux (e.g., per body mass, or per cell volume) is distinguished from oxygen flow (per number of objects, such as cells), IO2 [mol·s-1·x-1]. These are different forms of normalization of rate. |
| Oxygen flux - instrumental background | J°O2 | Instrumental background oxygen flux, J°O2, in a respirometer is due to oxygen consumption by the POS, and oxygen diffusion into or out of the aqueous medium in the O2k-chamber. It is a property of the instrumental system, measured in the range of experimental oxygen levels by a standardized instrumental O2 background test. The oxygen regime from air saturation towards zero oxygen is applied generally in experiments with isolated mitochondria, and living or permeabilized cells. To overcome oxygen diffusion limitation in permeabilized fibers and homogenates, an elevated oxygen regime is applied, requiring instrumental background test in the same range of elevated oxygen. |
| Oxygen pressure | pO2 [kPa] | Oxygen pressure or partial pressure of oxygen [kPa], related to oxygen concentration in solution by the oxygen solubility, SO2 [µM/kPa]. |
| Oxygen sensor test | POS test | The O2 sensor test is an important component of Oroboros Quality Management. The OroboPOS test is described in detail in MiPNet06.03 POS-calibration-SOP, is performed after switching on the Oroboros O2k, and is required as a basis of technical service of the instrument. |
| Oxygen solubility | SO2 [µM/kPa] | The oxygen solubility, SO2 [µM/kPa] = [(µmol·L-1)/kPa], expresses the oxygen concentration in solution in equilibrium with the oxygen pressure in a gas phase, as a function of temperature and composition of the solution. The inverse of oxygen solubility is related to the activity of dissolved oxygen. The oxygen solubility in solution, SO2(aq), depends on temperature and the concentrations of solutes in solution, whereas the dissolved oxygen concentration at equilibrium with air, cO2*(aq), depends on SO2(aq), barometric pressure and temperature. SO2(aq) in pure water is 10.56 µM/kPa at 37 °C and 12.56 µM/kPa at 25 °C. At standard barometric pressure (100 kPa), cO2*(aq) is 207.3 µM at 37 °C (19.6 kPa partial oxygen pressure) or 254.7 µM at 25 °C (20.3 kPa partial oxygen pressure). In MiR05 and serum, the corresponding saturation concentrations are lower due to the oxygen solubility factor: 191 and 184 µM at 37 °C or 234 and 227 µM at 25 °C. |
| Oxygen solubility factor | FM | The oxygen solubility factor of the incubation medium, FM, expresses the effect of the salt concentration on oxygen solubility relative to pure water. In mitochondrial respiration medium MiR05, MiR05-Kit and MiR06, FM is 0.92 (determined at 30 and 37 °C) and in culture media is 0.89 (at 37 °C). FM varies depending on the temperature and composition of the medium. To determine the FM based on the oxygen concentration, specific methods and equipment are needed (see references Rasmussen HN, Rasmussen UF 2003 in MiPNet06.03). For other media, FM may be estimated using Table 4 in MiPNet06.03. For this purpose KCl based media can be described as "seawater" of varying salinity. The original data on sucrose and KCl-media (Reynafarje et al 1985), however, have been critizesed as artefacts and the FM of 0.92 is suggested in the temperature range of 10 °C to 40 °C as for MiR05. |
| P-L control efficiency | jP-L | |
| P-L net OXPHOS capacity | P-L | |
| P/E control ratio | P/E | |
| P/O ratio | P/O ratio | P/O ratio stands for phosphate to atomic oxygen ratio, where P indicates phosphorylation of ADP to ATP (or GDP to GTP). |
| P50 | p50 | p50 is the oxygen partial pressure at which (a) respiratory flux is 50% of maximum oxygen flux, Jmax, at saturating oxygen levels. The oxygen affinity is indirectly proportional to the p50. The p50 depends on metabolic state and rate. (b) p50 is the oxygen partial pressure at which oxygen binding (on myoglobin, haemoglobin) is 50%, or desaturation is 50%. |
| PBMC | PBMC | Peripheral blood mononuclear cells (PBMC) are a fraction of the leucocyte population in the blood composed by cells with round nucleus. PBMC consist of lymphocytes (T, B and NK cells) and monocytes. During extraction, neutrophils and platelets (PLT) can be found in the PBMC fraction, where PLT are considered as a contamination. |
| PGM-pathway control state | PGM | PGM: Pyruvate & Glutamate & Malate.
MitoPathway control state: NADH electron transfer-pathway state Pyruvate (P) is oxidatively decarboxylated to acetyl-CoA and CO2, yielding NADH catalyzed by pyruvate dehydrogenase. Malate (M) is oxidized to oxaloacetate by mt-malate dehydrogenase located in the mitochondrial matrix. Condensation of oxaloacate with acetyl-CoA yields citrate (citrate synthase). Glutamate&malate is a substrate combination supporting an N-linked pathway control state, when glutamate is transported into the mt-matrix via the glutamate-aspartate carrier and reacts with oxaloacetate in the transaminase reaction to form aspartate and oxoglutarate. Glutamate as the sole substrate is transported by the electroneutral glutamate-/OH- exchanger, and is oxidized in the mitochondrial matrix by glutamate dehydrogenase to α-ketoglutarate ( 2-oxoglutarate), representing the glutamate-anaplerotic pathway control state. 2-oxoglutarate (α-ketoglutarate) is formed from isocitrate (isocitrate dehydrogenase, from oxaloacetate and glutamate by the transaminase, and from glutamate by the glutamate dehydrogenase. |
| PGMS-pathway control state | PGMS | PGMS: Pyruvate & Glutamate & Malate & Succinate.
MitoPathway control state: NS-pathway control state 2-oxoglutarate is produced through the citric acid cycle from citrate by isocitrate dehydrogenase, from oxaloacetate and glutamate by the transaminase, and from glutamate by the glutamate dehydrogenase. If the 2-oxoglutarate carrier does not outcompete these sources of 2-oxoglutarate, then the TCA cycle operates in full circle with external pyruvate&malate&glutamate&succinate |
| PGMSGp pathway control state | PGMSGp | PGMSGp: Pyruvate & Glutamate & Malate & Succinate & Glycerophosphate.
MitoPathway control state: NSGp SUIT protocol: SUIT-038 This substrate combination supports convergent electron flow to the Q-junction. |
| PH | pH | The pH value or pH is the negative of the base 10 logarithm of the activity of protons (hydrogen ions, H+). A pH electrode reports the pH and is sensitive to the activity of H+. In dilute solutions, the hydrogen ion activity is approximately equal to the hydrogen ion concentration. The symbol pH stems from the term potentia hydrogenii. |
| PM-pathway control state | PM | PM: Pyruvate & Malate.
MitoPathway control state: NADH Electron transfer-pathway state
|
| PMS-pathway control state | PMS | PMS: Pyruvate & Malate & Succinate.
MitoPathway control: CI&II Pyruvate (P) is oxidatively decarboxylated to acetyl-CoA and CO2, yielding NADH catalyzed by pyruvate dehydrogenase. Malate (M) is oxidized to oxaloacetate by mt-malate dehydrogenase located in the mitochondrial matrix. Condensation of oxaloacate with acetyl-CoA yields citrate (citrate synthase). This documents an additive effect of convergent CI&II electron flow to the Q-junction, with consistent results obtained with permeabilized muscle fibres and isolated mitochondria (Gnaiger 2009). |
| POS calibration - static | F5 | Two-point calibration of the polarographic oxygen sensor, comprising Air calibration and Zero calibration. See also POS calibration - dynamic. |
| POS-Membranes | FEP-membranes | POS-Membranes, FEP 25 µm; 40/Pck. |
| PalM | PalM | PalM: Palmitoylcarnitine & Malate.
MitoPathway control state: Fatty acid oxidation pathway control state SUIT protocols: SUIT-019 |
| PalOctM | PalOctM | PalOctM: Palmitoylcarnitine & Octanoylcarnitine & Malate.
MitoPathway control state: Fatty acid oxidation pathway control state SUIT protocols: SUIT-019 |
| PalOctPGM | PalOctPGM | PalOctPGM: Palmitoylcarnitine & Octanoylcarnitine & Pyruvate & Glutamate & Malate.
MitoPathway control state: FN SUIT protocols: SUIT-019 |
| PalOctPGMS | PalOctPGMS | PalOctPGMS: Palmitoylcarnitine & Octanoylcarnitine & Pyruvate & Glutamate & Malate & Succinate.
MitoPathway control state: FNS SUIT protocols: SUIT-019 |
| PalOctPM | PalOctPM | PalOctPM: Palmitoylcarnitine & Octanoylcarnitine & Pyruvate & Malate.
MitoPathway control state: FN SUIT protocols: SUIT-019 |
| PalPGMSGp pathway control state | PalPGMSGp | PalPGMSGp: Palmitoylcarnitine & Pyruvate & Glutamate & Malate & Succinate & Glycerophosphate.
MitoPathway control state: FNSGp SUIT protocol: SUIT-026 This substrate combination supports convergent electron flow to the Q-junction. |
| Palmitate | Paa | Palmitate is a term for the salts and esters of palmitic acid (CH3(CH2)14COOH). Palmitic acid is the first fatty acid produced during fatty acid synthesis and the precursor to longer fatty acids. Palmitate negatively feeds back on acetyl-CoA carboxylase (ACC), which is responsible for converting acetyl-CoA to malonyl-CoA, which in turn is used to add to the growing acyl chain, thus preventing further palmitate generation. In order to dissolve the water-insoluble sodium palmitate, BSA is needed to form the water-soluble compound called palmitate:BSA. |
| Palmitoyl-CoA | Pa-CoA | Palmitoyl-CoA is a coenzyme A derivative of palmitate formed by acyl-CoA synthase. In contrast to medium- and short-chain acyl-CoA, palmitoyl-CoA cannot freely diffuse into the mitochondrial matrix. Formation of palmitoylcarnitine by CPTI is necessary prior to transfer into mitochondria for further fatty acid oxidation (β-oxidation). To study Fatty acid oxidation using Palmitoyl-CoA, Carnitine and low amount of malate is needed on mitochondrial preparations. |
| Palmitoylcarnitine | Pal | Palmitoylcarnitine is an ester derivative of carnitine (long-chain acylcarnitine) involved in the metabolism of fatty acids. Within the cell, palmitoylcarnitine is transported into the mitochondria to deliver palmitate for fatty acid oxidation and energy production. |
| Partial oxygen pressure | pO2 [kPa] | The partial oxygen pressure pO2 [kPa] is the contribution of the O2 gas pressure to the total gas pressure. According to the gas law, the partial oxygen pressure is pO2(g) = nO2(g)·V·RT, where the concentration is cO2(g) = nO2(g)·V-1 [mol·m-3], R is the gas constant, and T is the absolute temperature, and RT is expressed in units of chemical force [J·mol-1]. In aqueous solutions at equilibrium with a gas phase, the partial O2 pressures are equal in the aqueous phase (aq) and gas phase (g), pO2(aq) = pO2(g) at total pressures where the partial pressure equals the fugacity. The O2 concentration in the aqueous phase, however, is much lower than in the gas phase, due to the low oxygen solubility in water. The activity of dissolved O2 is expressed by the pO2, where the solubility can be seen as an activity coefficient. |
| Particle charge | QNX, QNX | The particle charge QNX (QNX) or charge per elementary entity is the charge QelX [C] carried by ions of type X divided by the count NX [x]. The particle charge per proton is the elementary charge or proton charge e. |
| Pascal | Pa | The pascal [Pa] is the SI unit for pressure. [Pa] = [J·m-3] = [N·m-2] = [m-1·kg·s-2]. The standard pressure is 100 kPa = 1 bar (105 Pa; 1 kPa = 1000 Pa). Prior to 1982 the standard pressure has been defined as 101.325 kPa or 1 standard atmosphere (1 atm = 760 mmHg). |
| Pathway control efficiency | jZ-Y | Pathway control efficiencies are flux control efficiencies, expressing the relative change of flux in response to a transition between two electron-transfer-pathway states due to a change of (1) substrate availability or (2) inhibition of enzyme steps in the pathway, in a defined coupling-control state. |
| Pathway control ratio | FCR | Substrate control ratios are flux control ratios FCR, at a constant mitochondrial coupling-control state. Whereas there are only three well-defined coupling-control states of mitochondrial respiration, L, P, E (LEAK respiration, OXPHOS, Electron transfer pathway), numerous Electron-transfer-pathway states are possible. Careful selection of the reference state, Jref, is required, for which some guidelines may be provided without the possibility to formulate general rules. FCR are best defined by taking Jref as the maximum flux (e.g. NSE), such that flux in various other respiratory states, Ji, is smaller or equal to Jref. However, this is not generally possible with FCR. For instance, the N/S pathway control ratio (at constant coupling-control state) may be larger or smaller than 1.0, depending on the mitochondrial source and various mitochondrial injuries. The S-pathway control state may be selected preferentially as Jref, if mitochondria with variable N-linked injuries are studied. In contrast, the reference state, Z, is strictly defined for flux control efficiency. |
| Perfluorooctanoic acid | PFOA | Perfluorooctanoic acid (PFOA) is a metabolically inert perfluorinated fatty acid which activates UCP1 in brown-fat mitochondria. UCP1-dependent respiration can be stimulated with 600 μM PFOA after inhibition of the phosphorylation system. |
| Permeability transition pore | PTP | The (mitochondrial, mt) permeability transition pore (PTP) is an unspecific pore presumed to involve components of both the inner and outer mt membrane which upon opening induces a massive increase of the inner mt membrane permeability for solutes up to 1.5 kDa. It is crucially involved in cell death induction in response to, among other stimuli, radical stress and/or calcium overload and may cause necrosis or apoptosis. It plays an important role in neurodegenerative diseases, cardiac ischemia-reperfusion injury and possibly various other diseases. Previously considered essential molecular constituents such as the voltage-dependent anion channel (VDAC), the adenine nucleotide translocator (ANT) and cyclophilin D (CypD) have all been shown to be important regulators of mtPTP opening, but the molecular entities actually forming the pore are still unknown at present. The opening of the pore can be prevented using cyclosporin A, a compound that binds cyclophilin D avoiding the formation of the pore. In respirometry, mtPTP opening may be observed as a sudden decrease of respiration of isolated mitochondria (Hansson 2010 J Biol Chem). |
| Permeabilized cells | pce | Permeabilized cells (pce) are mitochondrial preparations obtained by selectively permeabilizing the plasma membrane (e.g., with digitonin), for the exchange of soluble molecules between the cytosolic phase and external medium, without damaging the mt-membranes.
Permeabilized cells (pce) are, therefore, not any longer viable or living cells (ce), since the intactness of cells implies the intactness of the plasma membrane. Any typical quantiative cell viability test (trypan blue etc) evaluating the intactness of the plasma membrane, yields a 100% negative result on fully permeabilized cells. For permeabilizing the cell plasma membranes chemically with digitonin, without damaging the mt-membranes, the optimum concentration of digitonin must be previously determinated. The protocol SUIT-010 is designed for the evaluation of optimum digitonin concentration for permeabilizing cells, a requirement to account for differences between cell types, the concentration of cells, and variability between batches of the natural product digitonin. |
| Permeabilized muscle fibers | pfi | Permeabilized muscle fibers (pfi) are used as a mitochondrial preparation in respirometry to access mitochondrial function comparable to isolated mitochondria (imt). pfi are obtained by selectively permeabilizing the plasma membrane mechanically and chemically (saponin), for the exchange of soluble molecules between the cytosolic phase and external medium, without damaging the mt-membranes.
|
| Permeabilized tissue | pti | Permeabilized tissue (pti, see also permeabilized muscle fibers, pfi) are mitochondrial preparations obtained by selectively permeabilizing the plasma membrane mechanically or chemically (e.g., with saponin), for the exchange of soluble molecules between the cytosolic phase and external medium, without damaging the mt-membranes. |
| Permeabilized tissue or cells | pti, pce | Permeabilized tissue (pti, see also permeabilized muscle fibers, pfi) or cells (pce) are mitochondrial preparations obtained by selectively permeabilizing the plasma membrane mechanically or chemically, for the exchange of soluble molecules between the cytosolic phase and external medium, without damaging the mt-membranes. Permeabilized cells (pce) are, therefore, not any longer viable or living cells (ce), since the intactness of cells implies the intactness of the plasma membrane. Any typical quantiative cell viability test (trypan blue etc) evaluating the intactness of the plasma membrane, yields a 100% negative result on fully permeabilized cells. |
| Peroxisome proliferator-activated receptor gamma coactivator 1-alpha | PGC-1α | Peroxisome proliferator-activated receptor-γ (PPAR-γ) coactivator-1α (PGC-1α) is a protein which functions as an inducible transcriptional coactivator, a coregulator of transcription factors, particularly NRF-1 and TFAM. PGC-1α was first described in 1998 (Puigserver 1998 Cell). PGC-1α drives the formation of slow-twich muscle fibres (Lin 2002 Nature) and is increased upon endurance training (Norrbom 2004 J Appl Physiol). PGC-1α expression is inhibited by the proinflammatory cytokine tumor necrosis factor α (TNF-α) and high levels of leptin. Upregulation of PGC-1α expression is induced by increased eNOS activity -> NO -> guanylate cyclase -> cGMP (Nisoli 2007 Circ Res). AMP-activated protein kinase (AMPK) increases PGC-1α expression through SIRT1 (Canto 2009 Nature). |
| Phosphate | Pi | See: Inorganic phosphate |
| Phosphate carrier | PiC | The phosphate carrier (PiC) is a proton/phosphate symporter which transports negatively charged inorganic phosphate across the inner mt-membrane. The transport can be described either as symport of H+ with Pi, or antiport of hydroxide anion against Pi. The phosphate carrier is a component of the phosphorylation system. |
| Phosphocreatine | PCr | Phosphocreatine is a high energy compound in the skeletal muscle of vertebrates and is present in 4 to 5 times the concentration of ATP. |
| Phosphoenolpyruvate carboxykinase | PEPCK | Phosphoenolpyruvate carboxykinase (PEPCK) catalyzes the anabolic reaction of oxaloacetate (Oxa) to phosphoenolpyruvate at the expense of GTP. PEPCK is a cytoplasmatic enzyme involved in gluconeogenesis in mouse and rat liver, but 'is found in the mitochondria in the rabbit and chicken, and in both cytoplasm and mitochondria in the guinea pig' (Lehninger 1970). In many anoxia-resistant animals, PEPCK plays an important catabolic role under severe hypoxia and anoxia at the PEPCK branchpoint (Hochachka, Somero 2002), feeding malate into the reversed TCA cycle: malate is dismutated to pyruvate catalyzed by malic enzyme in the oxidative direction, and to fumarate in the reductive direction, leading to formation of succinate and ATP under anoxia (Gnaiger 1977). |
| Phosphorylation pathway | DT | The phosphorylation pathway (phosphorylation system) is the functional unit utilizing the protonmotive force to phosphorylate ADP (D) to ATP (T), and may be defined more specifically as the P»-system. The P»-system consists of adenine nucleotide translocase, phosphate carrier, and ATP synthase. Mitochondrial adenylate kinase, mt-creatine kinase and mt-hexokinase constitute extended components of the P»-system, controlling local AMP and ADP concentrations and forming metabolic channels. Since substrate-level phosphorylation is involved in the TCA-cycle, the P»-system includes succinyl-CoA ligase (GDP to GTP or ADP to ATP). |
| PhotoBiology | PB | PhotoBiology is the science of the effect of light on biological processes. This includes photosynthesis, photochemistry, photophysics, photomorphogenesis, vision, bioluminescence, circadian rhythms and photodynamic therapy. Phototoxicity results from non-ionizing radiation (i.e. ultraviolet, visible and infrared radiation). Non-ionizing radiation is any type of electromagnetic radiation that does not carry enough energy per quantum (photon energy below 10 eV) to completely remove an electron from an atom or molecule. When photons interact with molecules, the molecules can absorb the photon energy and become excited, reacting with surrounding molecules and stimulating "photochemical" and "photophysical" changes. Respiration may be affected by light during photosynthesis or in dark respiration, with the transient response of light-enhanced dark respiration. |
| Photodecomposition | PD | Photodecomposition or photodegradation is the process of decay of organic material induced by increasing light intensity. Under aerobic conditions, the enhancement of photodecomposition by light intensity can be quantified by oxygen consumption in a controlled light regime. |
| Photosynthesis | PS | Photosynthesis is the process that converts light energy into chemical energy which is subsequently transformed to the physiological energy demand. Photosynthesis has a light-dependent and light-independent (dark) phase. In plants, algae, and cynobacteria, light energy is absorbed during the light phase by the pigment chlorophyll and used to split water and generate adenosine triphosphate (ATP) and reducing power - nicotinamide adenine dinucleotide phosphate (NADPH), with the net production of O2 as a waste product. During the dark phase ATP and NADPH are used to synthesize carbohydrates from CO2 through the metabolic pathway called Calvin-Benson cycle. Oxygenic photosynthesis is responsible for producing and maintaining the oxygen concentration of the Earth’s atmosphere. In bacteria such as cyanobacteria, photosynthesis involves the plasma membrane and the cytoplasm. In eukaryotic cells (plants and algae), photosynthesis takes place in the chloroplasts. |
| Plan S | S | Plan S is an initiative for Open Access publishing that was launched in September 2018. The plan is supported by cOAlition S, an international consortium of research funding and performing organisations. Plan S requires that, from 2021, scientific publications that result from research funded by public grants must be published in compliant Open Access journals or platforms. According to Science Europe, "Plan S requires that recipients of research funding from cOAlition S organisations make the resulting publications available immediately (without embargoes) and under open licences, either in quality Open Access platforms or journals or through immediate deposit in open repositories that fulfil the necessary conditions." |
| Platelet | PLT | Platelets or thrombocytes (PLT) are cell fragments derived from megakaryocytes with hemostatic function in the blood stream. PLT are anucleated but contain functioning mitochondria that play a critical role in PLT activation. |
| Platelet-rich plasma | PRP | Platelet-rich plasma (PRP) is obtained as the upper layer at low-speed centrifugation (around 150-200 g), when white and red blood cells sediment and thus get separated from plasma containing the platelets. For further details see blood cell preparation. |
| Plot - DatLab | Ctrl+F6 | A plot in DatLab represents a specific channel in the graph. To change the Layout for DatLab graphs go to [Graph]/Select plots to open the Graph layout window. |
| Polarization voltage | U | A polarization voltage of 600 mV to 800 mV is applied between anode and cathode of the polarographic oxygen sensor, resulting in a current when oxygen is consumed. The current is converted by the electronics to a voltage (raw signal) which must not be confused with the polarization voltage. |
| Polarographic oxygen sensor | POS | Polarographic oxygen sensors (POS) are operated with a polarization voltage between the cathode and anode, connected by an electrolyte. Cathode, anode and electrolyte are separated from the analyte by an oxygen-permeable membrane. Oxygen is reduced at the cathode such that the local oxygen concentration is maintained at zero, and diffuses along the concentration gradient from the stirred medium to the cathode, resulting in a linear calibration between oxygen partial pressure and electric current [Amp] (amperometric mode of operation). The OroboPOS is the POS applied in the Oroboros O2k. |
| Polyether ether ketone | PEEK | Polyether ether ketone (PEEK) is a semicrystalline organic polymer thermoplastic, which is chemically very resistant, with excellent mechanical properties. PEEK is compatible with ultra-high vacuum applications, and its resistance against oxygen diffusion make it an ideal material for high-resolution respirometry (POS insulation; coating of stirrer bars; stoppers for closing the O2k-Chamber). |
| Polyvinylidene fluoride | PVDF | Polyvinylidene fluoride (PVDF) is a pure thermoplastic fluoropolymer, which is chemically very resistant, with excellent mechanical properties. It is used generally in applications requiring the highest purity, strength, and resistance to solvents, acids, bases and heat (Wikipedia). PVDF is resistant against oxygen diffusion which makes it an ideal material for high-resolution respirometry (coating of stirrer bars; stoppers for closing the O2k-Chamber). |
| Power | P [W] | Power P [W = J·s-1] is exergy per time, or force times flow, which cannot be created internally yet is not conserved but is dissipated (P < 0) in irreversible energy transformations at constant temperature and (barometric) pressure, T,p. Metabolic power and heat flux of irreversible processes are distinguished as the time rate of Gibbs energy and enthalpy changes, respectively. |
| Pressure | p, P, Π [Pa] | Pressure is a fundamental quantity expressing energy per volume. The SI unit of pressure is generally pascal [Pa] = [J·m-3]. The term 'stress' (mechanical stress) is used as a synonym for pressure (SI). Pressure is known in physics as mechanical pressure, which is force per area, p = F·A-1 [Pa] = [N·m-2]. In physical chemistry, gas pressure is defined as p = n·V-1·RT, where the concentration is c = n·V-1 [mol·m-3], R is the gas constant, and T is the absolute temperature, and RT is expressed in units of chemical force [J·mol-1]. van't Hoff's osmotic pressure assumes the same form applied to dissolved substances diffusing across a semipermeable membrane, but concentrations should be replaced by activities. The activity of dissolved gases is expressed by the partial pressure, where the solubility can be seen as an activity coefficient. Pressure appears explicitely or implicitely in all chapters of physics and physical chemistry. In contrast to the universal counterparts energy and force, however, the general connections between various isomorphic expressions of pressure remain poorly understood: Pressure is the concentration of the force at the point of action. More generally, pressure is the force times concentration at the interphase of interaction. |
| Proficiency test | PT | Proficiency testing PT is an evaluation of participant performance against pre-established criteria by means of interlaboratory comparisons. Some PT providers in the medical area use the term “External Quality Assessment (EQA)” for their proficiency testing schemes, or for their broader programmes, or both. Internal PT strategies may be implemented into laboratory science as practical steps towards PT to achieve reproducibility. |
| Proline | Pro |
Proline (Pro), C5H9NO2, is an amino acid which occurs under physiological conditions mainly in the nonpolar form, with pKa1 = 1.99 pKa2 = 10.96. Proline is an anaplerotic substrate that supports both the proline pathway control state and the glutamate-anaplerotic pathway control state. Proline is used as a single substrate or in combination with carbohydrate-derived metabolites in mitochondria particularly of flight muscle of many (but not all) insects. Proline is oxidized to delta-1-pyrroline-5-carboxylate by the mtIM L-proline:quinone oxidoreductase (proline dehydrogenase, ProDH), with reduction of FAD to FADH2 and direct entry into the Q-junction. delta-1-pyrroline-5-carboxylate is converted to glutamate by 1-pyrroline-5-carboxylate dehydrogenase. |
| Proline dehydrogenase | ProDH | Proline dehydrogenase (ProDH), L-proline:quinone oxidoreductase, is located on the inner side of the mtIM, oxidizing proline to delta-1-pyrroline-5-carboxylate, with reduction of FAD to FADH2 and direct entry into the Q-junction, exerting an additive effect of convergent pathways. ProDH is widely distributed in a variety of organisms, is a source of ROS, and may play a role in carcinogenesis. |
| Proton | p+, p | The terms proton p and hydrogen ion H+ are used synonymously in chemistry. In particle physics, a proton is a subatomic particle with a positive electric charge. Protons and neutrons are collectively referred to as nucleons. The proton is a bare charge with only about 1/64 000 of the radius of a hydrogen atom, and so the free proton is extremely reactive chemically. Therefore, the free proton has an extremely short lifetime in aqueous solutions where it forms the hydronium ion, H3O+, which in turn is further solvated by water molecules in clusters such as H5O2+ and H9O4+. |
| Protonmotive force | pmF, ∆mFH+, Δp [J·MU-1] | The protonmotive force ∆mFH+ is known as Δp in Peter Mitchell’s chemiosmotic theory [1], which establishes the link between electric and chemical components of energy transformation and coupling in oxidative phosphorylation. The unifying concept of the pmF ranks among the most fundamental theories in biology. As such, it provides the framework for developing a consistent theory and nomenclature for mitochondrial physiology and bioenergetics. The protonmotive force is not a vector force as defined in physics. This conflict is resolved by the generalized formulation of isomorphic, compartmental forces, ∆trF, in energy (exergy) transformations [2]. Protonmotive means that there is a potential for the movement of protons, and force is a measure of the potential for motion.
The pmF is generated in oxidative phosphorylation by oxidation of reduced fuel substrates and reduction of O2 to H2O, driving the coupled proton translocation from the mt-matrix space across the mitochondrial inner membrane (mtIM) through the proton pumps of the electron transfer pathway (ETS), which are known as respiratory Complexes CI, CIII and CIV. ∆mFH+ consists of two partial isomorphic forces: (1) The chemical part, ∆dFH+, relates to the diffusion (d) of uncharged particles and contains the chemical potential difference§ in H+, ∆µH+, which is proportional to the pH difference, ∆pH. (2) The electric part, ∆elFp+ (corresponding numerically to ∆Ψ)§, is the electric potential difference§, which is not specific for H+ and can, therefore, be measured by the distribution of any permeable cation equilibrating between the negative (matrix) and positive (external) compartment. Motion is relative and not absolute (Principle of Galilean Relativity); likewise there is no absolute potential, but isomorphic forces are stoichiometric potential differences§. The total motive force (motive = electric + chemical) is distinguished from the partial components by subscript ‘m’, ∆mFH+. Reading this symbol by starting with the proton, it can be seen as pmF, or the subscript m (motive) can be remembered by the name of Mitchell, ∆mFH+ = ∆dFH+ + ∆elFp+ With classical symbols, this equation contains the Faraday constant, F, multiplied implicitly by the charge number of the proton (zH+ = 1), and has the form [1] ∆p = ∆µH+∙F-1 + ∆ΨA partial electric force of 0.2 V in the electrical format, ∆elFeH+a, is 19 kJ∙mol-1 H+a in the molar format, ∆elFnp+a. For 1 unit of ∆pH, the partial chemical force changes by -5.9 kJ∙mol-1 in the molar format, ∆dFnH+a, and by 0.06 V in the electrical format, ∆dFeH+a. Considering a driving force of -470 kJ∙mol-1 O2 for oxidation, the thermodynamic limit of the H+a/O2 ratio is reached at a value of 470/19 = 24, compared to the mechanistic stoichiometry of 20 for the N-pathway with three coupling sites. |
| Protonmotive pressure | pmP, ∆mΠH+ [kPa] | The protonmotive pressure, ∆mΠH+ or pmP [kPa], is an extension of Peter Mitchell’s concept of the protonmotive force pmF, based on Fick’s law of diffusion and Einstein’s diffusion equation, accounting for osmotic pressure (corresponding to the diffusion term in the pmF) and electric pressure (the electric term or membrane potential in the pmF). The linearity of the generalized flow-pressure relationship explains the non-ohmic flow-force dependence in the proton leak rate as a function of membrane potential.
The total motive pressure (motive = electric + chemical) is distinguished from the partial components by subscript ‘m’, ∆mΠH+, ∆mΠH+ = ∆dΠH+ + ∆elΠp+ |
| Publication efficiency | Fr,a/p | Publication efficiency is the fraction Fr,a/p of reproducible publications Nr which are among the number Na of publications that receive attention and meaningful interpretation, per total count Np of all published communications. Publication efficiency Fr,a/p = Fr/p·Fa/p is low due to (1) the reproducibility crisis expressed as low reproducibility efficiency Fr/p = Nr/Np, and (2) the inflation crisis expressed as low attention efficiency Fa/p = Na/Np. Estimates of these partial efficiencies vary from field to field. With Fr/p=0.15 and Fa/p=0.05, the current publication efficiency is as low as 0.0075, or only 0.75 % of all presently published communictions are reproducible and receive attention and meaningful interpretation. Reduction of the number of irreproducible zero-value publications is the most effective measure to reduce the paper mass excess (PME) in the reproducibility-inflation (R&I)-crisis. Several regulatory mechanisms for improvement are practically ignored although theoretically available. |
| Publicly deposited protocols | PDP | Researchers need to be introduced into adhering to publicly deposited protocols. Prespecified and time-stamped protocols that are publicly deposited may help to save Millions of Euros that may otherwise be wasted on research that is lacking coherent standards. |
| Pyruvate | P | Pyruvic acid, C3H4O3, is an alpha-keto monocarboxylic acid which occurs under physiological conditions mainly as the anion pyruvate-, P, with pKa = 2.5. Pyruvate is formed in glycolysis from phosphoenolpyruvate. In the cytosol, pyruvate is a substrate of lactate dehydrogenase. Pyruvate enters the mitochondrial matrix via a specific low Km' H+/monocarboxylate cotransporter known as the pyruvate carrier. Similarly, the plasma membrane of many cell types has H+/monocarboxylate cotransporter activity and pyruvate can thus be added as a substrate to living cells. In the mt-matrix the oxidative decarboxylation of pyruvate is catalyzed by pyruvate dehydrogenase and yields acetyl-CoA. Pyruvate competitively reverses the inhibition of cytochrome c oxidase by cyanide. Pyruvate is an antioxidant reacting with hydrogen peroxide. |
| Pyruvate carboxylase | PC | Pyruvate carboxylase synthesizes oxaloacetate from pyruvate and CO2 as an anaplerotic reaction in the mitochondrial matrix of the liver and kidney of higher animals, representing an alternative to the malic enzyme pathway to oxaloacetate or the phosphoenolpyruvate carboxykinase reaction (compare glyoxylate cycle in plants and microorganisms). Carboxylation of pyruvate to oxaloacetate requires Mg-ATP. Acetyl CoA is a strong positive modulator. PC can form pyruvate from oxaloacetate to remove an excess of oxaloacetate which inhibits succinate dehydrogenase. |
| Pyruvate dehydrogenase | PDH | Pyruvate dehydrogenase is the first component enzyme of the pyruvate dehydrogenase complex, which catalyzes oxidative decarboxylation of pyruvate in the mt-matrix, and yields acetyl-CoA. PDH is known as the mitochondrial gatekeeper in the core energy pathway of electron flow into the tricarboxylic acid cycle. |
| Pyruvate dehydrogenase complex | PDHC | Oxidative decarboxylation of pyruvate is catalyzed by the pyruvate dehydrogenase complex in the mt-matrix, and yields acetyl-CoA. |
| P»-system | P»system | The ADP-ATP phosphorylation system or P»-system. See Phosphorylation system. |
| Q | Q | Multiple meanings of Q
|
| Q-Module | Q-Module | The Q-Module, developed for measuring the Q redox state and cyclic voltammetry, is supported by the NextGen-O2k and consists of the Q-Sensor, integrated electronic components in the O2k, and the DatLab software. |
| Q-cycle | Q | Q-cycle refers to the sequential oxidation and reduction of the electron carrier Coenzyme Q (CoQ or ubiquinone) in mitochondria or plastoquinones in the photosynthetic system. Originally, the concept of the Q-cycle was proposed by Peter D Mitchell. Following several modifications, the Q-cycle is established, describing how CIII translocates hydrogen ions against the protonmotive force. The reduced CoQ (ubiquinol QH2) binds to the Qo site of CIII, while the oxidized CoQ (ubiquinone Q) to the Qi site of CIII. First, QH2 reduces the iron-sulfur protein and feeds cytochrome c1 with one electron. The other electron is transferred to the bL heme and reduces the bH heme, which transfers the electron to ubiquinone at the Qi-site which is reduced to a semiquinone. A second QH2 is required to fully reduce semiquinone to ubiquinol. At the end of the Q-cycle, four protons leave the mt-matrix and enter the intermembrane space, and the reduced cytochrome c transfers electrons to CIV. The ubiquinol generated at the Qi-site can be reused by binding to the Qo-site of CIII. |
| Q-pools | Q | Different Q-pools are more or less clearly distinguished in the cell, related to a variety of models describing degress of Q-pool behavior. (1) CoQ-pools are distinguished according to their compartmentation in the cell: mitochondrial CoQ (mtCoQ) and CoQ in other organelles versus plasma-membrane CoQ. (2) The total mitochondrial CoQ-pool mtCoQ is partitioned into an ETS-reactive Q-pool, Qra, and an inactive mtCoQ-pool, Qia. (2a) The Qra-pool is fully reduced in the form of quinol QH2 under anoxia, and fully oxidized in the form of quinone in aerobic mitochondrial preparations incubated without CHNO-fuel substrates. Intermediate redox states of Qra are sensitive to pathway control and coupling control of mitochondrial electron transfer and OXPHOS. (2b) The Qia-pool remains partially reduced and oxidized independent of aerobic-anoxic transitions. The redox state of Qia is insensitive to changes in mitochondrial respiratory states. (3) The Qra-pool is partitioned into Q with Q-pool behavior according to the fluid-state model (synonymous: random-collision model) and Q tightly bound to supercomplexes according to the solid-state model. The two models describe the extremes in a continuum of homogenous or heterogenous Q-pool behavior. The CII-Q-CIII segment of the S-pathway is frequently considered to follow homogenous Q-pool behavior participating in the Qhom-pool, whereas the CI-Q-CIII segment of the N-pathway indicates supercomplex organization and metabolic channeling with different degrees of Q-pool heterogeneity contributing to the Qhet-pool. |
| Quantity | Q | A quantity is the attribute of a phenomenon, body or substance that may be distinguished qualitatively and determined quantitatively. A dimensional quantity is a number (variable, parameter, or constant) connected to its dimension, which is different from 1. {Quote} The value of a quantity is generally expressed as the product of a number and a unit. The unit is simply a particular example of the quantity concerned which is used as a reference, and the number is the ratio of the value of the quantity to the unit. {end of Quote: Bureau International des Poids et Mesures 2019 The International System of Units (SI), p. 127)}. |
| R-L control efficiency | jR-L | |
| R-L net ROUTINE capacity | R-L | |
| R/E control ratio | R/E | |
| ... further results | ||
References
- Gnaiger E (1993) Nonequilibrium thermodynamics of energy transformations. Pure Appl Chem 65: 1983-2002. >>Open Access
- Cohen ER, Cvitas T, Frey JG, Holmström B, Kuchitsu K, Marquardt R, Mills I, Pavese F, Quack M, Stohner J, Strauss HL, Takami M, Thor HL (2008) Quantities, Units and Symbols in Physical Chemistry. IUPAC Green Book, 3rd Edition, 2nd Printing, IUPAC & RSC Publishing, Cambridge. >>Open Access
- Gnaiger E (2012) Mitochondrial Pathways and Respiratory Control. An Introduction to OXPHOS Analysis. 3rd ed. Mitochondr Physiol Network 17.18. OROBOROS MiPNet Publications, Innsbruck: 64 pp. >>Open Access